The Biogenesis Process of VDAC - From Early Cytosolic Events to Its Final Membrane Integration
- PMID: 34456757
- PMCID: PMC8388839
- DOI: 10.3389/fphys.2021.732742
The Biogenesis Process of VDAC - From Early Cytosolic Events to Its Final Membrane Integration
Abstract
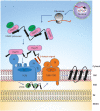
Voltage dependent anion-selective channel (VDAC) is the most abundant protein in the mitochondrial outer membrane. It is a membrane embedded β-barrel protein composed of 19 mostly anti-parallel β-strands that form a hydrophilic pore. Similar to the vast majority of mitochondrial proteins, VDAC is encoded by nuclear DNA, and synthesized on cytosolic ribosomes. The protein is then targeted to the mitochondria while being maintained in an import competent conformation by specific cytosolic factors. Recent studies, using yeast cells as a model system, have unearthed the long searched for mitochondrial targeting signal for VDAC and the role of cytosolic chaperones and mitochondrial import machineries in its proper biogenesis. In this review, we summarize our current knowledge regarding the early cytosolic stages of the biogenesis of VDAC molecules, the specific targeting of VDAC to the mitochondrial surface, and the subsequent integration of VDAC into the mitochondrial outer membrane by the TOM and TOB/SAM complexes.
Keywords: TOM complex; VDAC; beta-barrels; chaperones; mitochondria; outer membrane.
Copyright © 2021 Moitra and Rapaport.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bausewein T., Mills D. J., Langer J. D., Nitschke B., Nussberger S., Kühlbrandt W. (2017). Cryo-EM structure of the TOM core complex from Neurospora crassa. Cell 170 693–700.e697. - PubMed
-
- Benz R. (1989). “Porins from mitochondrial and bacterial outer membranes: structural and functional aspects,” in Anion Carriers of Mitochondrial Membranes, eds Azzi A., Nałęz K. A., Nałęcz M. J., Wojtczak L. (Berlin: Springer; ).
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

