Temporal Changes in the Function of Bacterial Assemblages Associated With Decomposing Earthworms
- PMID: 34456883
- PMCID: PMC8386022
- DOI: 10.3389/fmicb.2021.682224
Temporal Changes in the Function of Bacterial Assemblages Associated With Decomposing Earthworms
Abstract
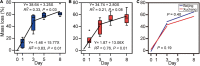
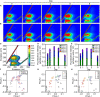
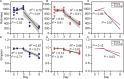
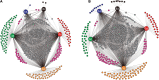
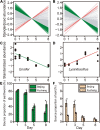
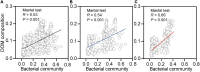
Soil invertebrate corpse decomposition is an ecologically significant, yet poorly understood, process affecting nutrient biogeochemical cycling in terrestrial ecosystems. Here, we attempted to answer how the substrate chemistry and microbial community change during soil invertebrate (earthworm) decomposition and what roles microbes play in this process. Specifically, the dead earthworms (Amynthas corticis) were buried in two soils where the earthworms inhabited, or not, until more than 50% of the earthworm mass was lost. For both soils, earthworms decomposed faster during the early stage (between 0 and 3 days), as reflected by the higher rate of decomposition and increased accumulation of dissolved organic matter (DOM). This decomposition pattern was paralleled by bacterial community dynamics, where bacterial richness and diversity were significantly higher during early decomposition (p < 0.05) with the relative abundances of many genera decreasing as decomposition progressed. The succession of the bacterial community composition was significantly correlated with time-course changes in DOM composition (p < 0.05). Particularly, more functional groups (e.g., microbes associated with carbon, nitrogen, and sulfur cycling) were identified to be linked with the change of a specific DOM type during the early decomposition phase. By exploring the ecologically important process of soil invertebrate decomposition and its associated bacterial communities, this study provides evidence, e.g., a statistically significant positive correlation between bacterial community and DOM compositions, which supports the widely recognized yet less-tested microbial community structure-function relationship hypothesis in invertebrate decomposition.
Keywords: bacterial community; decomposition; dissolved organic matter; earthworm; structure–function relationship.
Copyright © 2021 Sun and Ge.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Bastian M., Heymann S., Jacomy M. (2009). Gephi: an open source software for exploring and manipulating networks. Proc. Third Int. ICWSM Conf. 8 361–362.
-
- Benbow M. E., Barton P. S., Ulyshen M. D., Beasley J. C., DeVault T. L., Strickland M. S., et al. (2019). Necrobiome framework for bridging decomposition ecology of autotrophically and heterotrophically derived organic matter. Ecol. Monogr. 89:e01331.
-
- Benbow M. E., Receveur J. P., Lamberti G. A. (2020). Death and decomposition in aquatic ecosystems. Front. Ecol. Evol. 8:17. 10.3389/fevo.2020.00017 - DOI
LinkOut - more resources
Full Text Sources

