The Antimicrobial Activity of the AGXX® Surface Coating Requires a Small Particle Size to Efficiently Kill Staphylococcus aureus
- PMID: 34456898
- PMCID: PMC8387631
- DOI: 10.3389/fmicb.2021.731564
The Antimicrobial Activity of the AGXX® Surface Coating Requires a Small Particle Size to Efficiently Kill Staphylococcus aureus
Abstract
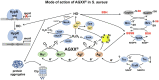
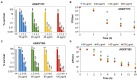
Methicillin-resistant Staphylococcus aureus (MRSA) isolates are often resistant to multiple antibiotics and pose a major health burden due to limited treatment options. The novel AGXX® surface coating exerts strong antimicrobial activity and successfully kills multi-resistant pathogens, including MRSA. The mode of action of AGXX® particles involves the generation of reactive oxygen species (ROS), which induce an oxidative and metal stress response, increased protein thiol-oxidations, protein aggregations, and an oxidized bacillithiol (BSH) redox state in S. aureus. In this work, we report that the AGXX® particle size determines the effective dose and time-course of S. aureus USA300JE2 killing. We found that the two charges AGXX®373 and AGXX®383 differ strongly in their effective concentrations and times required for microbial killing. While 20-40 μg/ml AGXX®373 of the smaller particle size of 1.5-2.5 μm resulted in >99.9% killing after 2 h, much higher amounts of 60-80 μg/ml AGXX®383 of the larger particle size of >3.2 μm led to a >99% killing of S. aureus USA300JE2 within 3 h. Smaller AGXX® particles have a higher surface/volume ratio and therefore higher antimicrobial activity to kill at lower concentrations in a shorter time period compared to the larger particles. Thus, in future preparations of AGXX® particles, the size of the particles should be kept at a minimum for maximal antimicrobial activity.
Keywords: AGXX®; Staphylococcus aureus; antimicrobial activity; contact killing; metal particles.
Copyright © 2021 Linzner and Antelmann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The AGXX® Antimicrobial Coating Causes a Thiol-Specific Oxidative Stress Response and Protein S-bacillithiolation in Staphylococcus aureus.Front Microbiol. 2018 Dec 11;9:3037. doi: 10.3389/fmicb.2018.03037. eCollection 2018. Front Microbiol. 2018. PMID: 30619128 Free PMC article.
-
Transcriptomic analysis of stress response to novel antimicrobial coatings in a clinical MRSA strain.Mater Sci Eng C Mater Biol Appl. 2021 Feb;119:111578. doi: 10.1016/j.msec.2020.111578. Epub 2020 Sep 30. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 33321624
-
Thiol-based redox switches in the major pathogen Staphylococcus aureus.Biol Chem. 2020 Nov 23;402(3):333-361. doi: 10.1515/hsz-2020-0272. Print 2021 Feb 23. Biol Chem. 2020. PMID: 33544504 Review.
-
Biofilm Forming Antibiotic Resistant Gram-Positive Pathogens Isolated From Surfaces on the International Space Station.Front Microbiol. 2019 Mar 19;10:543. doi: 10.3389/fmicb.2019.00543. eCollection 2019. Front Microbiol. 2019. PMID: 30941112 Free PMC article.
-
Application of genetically encoded redox biosensors to measure dynamic changes in the glutathione, bacillithiol and mycothiol redox potentials in pathogenic bacteria.Free Radic Biol Med. 2018 Nov 20;128:84-96. doi: 10.1016/j.freeradbiomed.2018.02.018. Epub 2018 Feb 15. Free Radic Biol Med. 2018. PMID: 29454879 Review.
Cited by
-
A novel silver-ruthenium-based antimicrobial kills Gram-negative bacteria through oxidative stress-induced macromolecular damage.mSphere. 2025 Jun 25;10(6):e0001725. doi: 10.1128/msphere.00017-25. Epub 2025 May 30. mSphere. 2025. PMID: 40444966 Free PMC article.
-
A novel ruthenium-silver based antimicrobial potentiates aminoglycoside activity against Pseudomonas aeruginosa.mSphere. 2023 Oct 24;8(5):e0019023. doi: 10.1128/msphere.00190-23. Epub 2023 Aug 30. mSphere. 2023. PMID: 37646510 Free PMC article.
-
Reactive Oxygen Species (ROS)-Mediated Antibacterial Oxidative Therapies: Available Methods to Generate ROS and a Novel Option Proposal.Int J Mol Sci. 2024 Jun 29;25(13):7182. doi: 10.3390/ijms25137182. Int J Mol Sci. 2024. PMID: 39000290 Free PMC article. Review.
-
A Novel Silver-Ruthenium-Based Antimicrobial Kills Gram-Negative Bacteria Through Oxidative Stress-Induced Macromolecular Damage.bioRxiv [Preprint]. 2025 Jan 4:2025.01.03.631245. doi: 10.1101/2025.01.03.631245. bioRxiv. 2025. Update in: mSphere. 2025 Jun 25;10(6):e0001725. doi: 10.1128/msphere.00017-25. PMID: 39803548 Free PMC article. Updated. Preprint.
-
Antimicrobials Functioning through ROS-Mediated Mechanisms: Current Insights.Microorganisms. 2021 Dec 28;10(1):61. doi: 10.3390/microorganisms10010061. Microorganisms. 2021. PMID: 35056511 Free PMC article. Review.
References
-
- Boudreau M. D., Imam M. S., Paredes A. M., Bryant M. S., Cunningham C. K., Felton R. P., et al. . (2016). Differential effects of silver nanoparticles and silver ions on tissue accumulation, distribution, and toxicity in the Sprague Dawley rat following daily oral gavage administration for 13 weeks. Toxicol. Sci. 150, 131–160. 10.1093/toxsci/kfv318, PMID: - DOI - PMC - PubMed
-
- Chen J.-Y., Wang L.-Y., Wu P.-W. (2010). Preparation and characterization of ruthenium films via an electroless deposition route. Thin Solid Films 518, 7245–7248. 10.1016/j.tsf.2010.04.086 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

