The Functions of Hepatitis B Virus Encoding Proteins: Viral Persistence and Liver Pathogenesis
- PMID: 34456908
- PMCID: PMC8387624
- DOI: 10.3389/fimmu.2021.691766
The Functions of Hepatitis B Virus Encoding Proteins: Viral Persistence and Liver Pathogenesis
Abstract
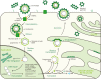
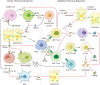
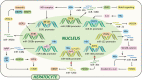
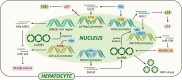
About 250 million people worldwide are chronically infected with Hepatitis B virus (HBV), contributing to a large burden on public health. Despite the existence of vaccines and antiviral drugs to prevent infection and suppress viral replication respectively, chronic hepatitis B (CHB) cure remains a remote treatment goal. The viral persistence caused by HBV is account for the chronic infection which increases the risk for developing liver cirrhosis and hepatocellular carcinoma (HCC). HBV virion utilizes various strategies to escape surveillance of host immune system therefore enhancing its replication, while the precise mechanisms involved remain elusive. Accumulating evidence suggests that the proteins encoded by HBV (hepatitis B surface antigen, hepatitis B core antigen, hepatitis B envelope antigen, HBx and polymerase) play an important role in viral persistence and liver pathogenesis. This review summarizes the major findings in functions of HBV encoding proteins, illustrating how these proteins affect hepatocytes and the immune system, which may open new venues for CHB therapies.
Keywords: encoding proteins; hepatitis B virus (HBV); hepatocellular carcinoma (HCC); immune response; viral escape.
Copyright © 2021 Zhao, Xie, Tan, Yu, Tian, Lv, Qin, Qi and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- World Health Organization . Global Hepatitis Report. (2017). Available at: https://www.who.int/publications/i/item/global-hepatitis-report-2017 (Accessed November 10, 2020).
-
- Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet (2018) 392(10159):1736–88. 10.1016/s0140-6736(18)32203-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

