Macrophages: The Good, the Bad, and the Gluttony
- PMID: 34456917
- PMCID: PMC8397413
- DOI: 10.3389/fimmu.2021.708186
Macrophages: The Good, the Bad, and the Gluttony
Abstract
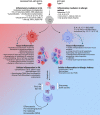
Macrophages are dynamic cells that play critical roles in the induction and resolution of sterile inflammation. In this review, we will compile and interpret recent findings on the plasticity of macrophages and how these cells contribute to the development of non-infectious inflammatory diseases, with a particular focus on allergic and autoimmune disorders. The critical roles of macrophages in the resolution of inflammation will then be examined, emphasizing the ability of macrophages to clear apoptotic immune cells. Rheumatoid arthritis (RA) is a chronic autoimmune-driven spectrum of diseases where persistent inflammation results in synovial hyperplasia and excessive immune cell accumulation, leading to remodeling and reduced function in affected joints. Macrophages are central to the pathophysiology of RA, driving episodic cycles of chronic inflammation and tissue destruction. RA patients have increased numbers of active M1 polarized pro-inflammatory macrophages and few or inactive M2 type cells. This imbalance in macrophage homeostasis is a main contributor to pro-inflammatory mediators in RA, resulting in continual activation of immune and stromal populations and accelerated tissue remodeling. Modulation of macrophage phenotype and function remains a key therapeutic goal for the treatment of this disease. Intriguingly, therapeutic intervention with glucocorticoids or other DMARDs promotes the re-polarization of M1 macrophages to an anti-inflammatory M2 phenotype; this reprogramming is dependent on metabolic changes to promote phenotypic switching. Allergic asthma is associated with Th2-polarised airway inflammation, structural remodeling of the large airways, and airway hyperresponsiveness. Macrophage polarization has a profound impact on asthma pathogenesis, as the response to allergen exposure is regulated by an intricate interplay between local immune factors including cytokines, chemokines and danger signals from neighboring cells. In the Th2-polarized environment characteristic of allergic asthma, high levels of IL-4 produced by locally infiltrating innate lymphoid cells and helper T cells promote the acquisition of an alternatively activated M2a phenotype in macrophages, with myriad effects on the local immune response and airway structure. Targeting regulators of macrophage plasticity is currently being pursued in the treatment of allergic asthma and other allergic diseases. Macrophages promote the re-balancing of pro-inflammatory responses towards pro-resolution responses and are thus central to the success of an inflammatory response. It has long been established that apoptosis supports monocyte and macrophage recruitment to sites of inflammation, facilitating subsequent corpse clearance. This drives resolution responses and mediates a phenotypic switch in the polarity of macrophages. However, the role of apoptotic cell-derived extracellular vesicles (ACdEV) in the recruitment and control of macrophage phenotype has received remarkably little attention. ACdEV are powerful mediators of intercellular communication, carrying a wealth of lipid and protein mediators that may modulate macrophage phenotype, including a cargo of active immune-modulating enzymes. The impact of such interactions may result in repair or disease in different contexts. In this review, we will discuss the origin, characterization, and activity of macrophages in sterile inflammatory diseases and the underlying mechanisms of macrophage polarization via ACdEV and apoptotic cell clearance, in order to provide new insights into therapeutic strategies that could exploit the capabilities of these agile and responsive cells.
Keywords: apoptosis; arthritis (including rheumatoid arthritis); asthma; inflammation; macrophage.
Copyright © 2021 Ross, Devitt and Johnson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

