Different Aspects of Classical Pathway Overactivation in Patients With C3 Glomerulopathy and Immune Complex-Mediated Membranoproliferative Glomerulonephritis
- PMID: 34456924
- PMCID: PMC8386118
- DOI: 10.3389/fimmu.2021.715704
Different Aspects of Classical Pathway Overactivation in Patients With C3 Glomerulopathy and Immune Complex-Mediated Membranoproliferative Glomerulonephritis
Abstract
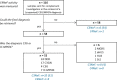
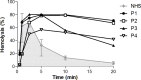
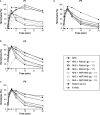
The rare and heterogeneous kidney disorder C3 glomerulopathy (C3G) is characterized by dysregulation of the alternative pathway (AP) of the complement system. C3G is often associated with autoantibodies stabilizing the AP C3 convertase named C3 nephritic factors (C3NeF). The role of classical pathway (CP) convertase stabilization in C3G and related diseases such as immune complex-mediated membranoproliferative glomerulonephritis (IC-MPGN) remains largely unknown. Here, we investigated the CP convertase activity in patients with C3G and IC-MPGN. Using a refined two-step hemolytic assay, we measured the stability of CP convertases directly in the serum of 52 patients and 17 healthy controls. In four patients, CP convertase activity was prolonged compared to healthy controls, i.e. the enzymatic complex was stabilized. In three patients (2 C3G, 1 IC-MPGN) the convertase stabilization was caused by immunoglobulins, indicating the presence of autoantibodies named C4 nephritic factors (C4NeFs). Importantly, the assay also enabled detection of non-immunoglobulin-mediated stabilization of the CP convertase in one patient with C3G. Prolonged CP convertase activity coincided with C3NeF activity in all patients and for up to 70 months of observation. Crucially, experiments with C3-depleted serum showed that C4NeFs stabilized the CP C3 convertase (C4bC2a), that does not contain C3NeF epitopes. All patients with prolonged CP convertase activity showed clear signs of complement activation, i.e. lowered C3 and C5 levels and elevated levels of C3d, C3bc, C3bBbP, and C5b-9. In conclusion, this work provides new insights into the diverse aspects and (non-)immunoglobulin nature of factors causing CP convertase overactivity in C3G/IC-MPGN.
Keywords: C3 convertase; C3 glomerulopathy; C3 nephritic factors (C3NeFs); C4 nephritic factors (C4NeFs); autoantibody; classical complement pathway.
Copyright © 2021 Michels, van de Kar, van Kraaij, Sarlea, Gracchi, Engels, Dorresteijn, van der Deure, Duineveld, Wetzels, van den Heuvel and Volokhina.
Conflict of interest statement
JW has received grants from Achillion and Chemocentryx, outside the submitted work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Goodship TH, Cook HT, Fakhouri F, Fervenza FC, Fremeaux-Bacchi V, Kavanagh D, et al. . Atypical Hemolytic Uremic Syndrome and C3 Glomerulopathy: Conclusions From a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int (2017) 91(3):539–51. 10.1016/j.kint.2016.10.005 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

