Hijacking of the Host's Immune Surveillance Radars by Burkholderia pseudomallei
- PMID: 34456925
- PMCID: PMC8384953
- DOI: 10.3389/fimmu.2021.718719
Hijacking of the Host's Immune Surveillance Radars by Burkholderia pseudomallei
Abstract
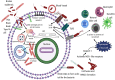
Burkholderia pseudomallei (B. pseudomallei) causes melioidosis, a potentially fatal disease for which no licensed vaccine is available thus far. The host-pathogen interactions in B. pseudomallei infection largely remain the tip of the iceberg. The pathological manifestations are protean ranging from acute to chronic involving one or more visceral organs leading to septic shock, especially in individuals with underlying conditions similar to COVID-19. Pathogenesis is attributed to the intracellular ability of the bacterium to 'step into' the host cell's cytoplasm from the endocytotic vacuole, where it appears to polymerize actin filaments to spread across cells in the closer vicinity. B. pseudomallei effectively evades the host's surveillance armory to remain latent for prolonged duration also causing relapses despite antimicrobial therapy. Therefore, eradication of intracellular B. pseudomallei is highly dependent on robust cellular immune responses. However, it remains ambiguous why certain individuals in endemic areas experience asymptomatic seroconversion, whereas others succumb to sepsis-associated sequelae. Here, we propose key insights on how the host's surveillance radars get commandeered by B. pseudomallei.
Keywords: Burkholderia pseudomallei; immunology; melioidosis; pathogenesis; virulence.
Copyright © 2021 Mariappan, Vellasamy, Barathan, Girija, Shankar and Vadivelu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

