Correction of Vertebral Bone Development in Ectodysplasin A1-Deficient Mice by Prenatal Treatment With a Replacement Protein
- PMID: 34456978
- PMCID: PMC8385758
- DOI: 10.3389/fgene.2021.709736
Correction of Vertebral Bone Development in Ectodysplasin A1-Deficient Mice by Prenatal Treatment With a Replacement Protein
Abstract
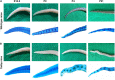
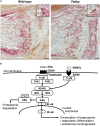
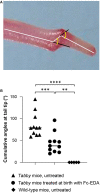
X-linked hypohidrotic ectodermal dysplasia with the cardinal symptoms hypodontia, hypotrichosis and hypohidrosis is caused by a genetic deficiency of ectodysplasin A1 (EDA1). Prenatal EDA1 replacement can rescue the development of skin appendages and teeth. Tabby mice, a natural animal model of EDA1 deficiency, additionally feature a striking kink of the tail, the cause of which has remained unclear. We studied the origin of this phenomenon and its response to prenatal therapy. Alterations in the distal spine could be noticed soon after birth, and kinks were present in all Tabby mice by the age of 4 months. Although their vertebral bones frequently had a disorganized epiphyseal zone possibly predisposing to fractures, cortical bone density was only reduced in vertebrae of older Tabby mice and even increased in their tibiae. Different availability of osteoclasts in the spine, which may affect bone density, was ruled out by osteoclast staining. The absence of hair follicles, a well-known niche of epidermal stem cells, and much lower bromodeoxyuridine uptake in the tail skin of 9-day-old Tabby mice rather suggest the kink being due to a skin proliferation defect that prevents the skin from growing as fast as the skeleton, so that caudal vertebrae may be squeezed and bent by a lack of skin. Early postnatal treatment with EDA1 leading to delayed hair follicle formation attenuated the kink, but did not prevent it. Tabby mice born after prenatal administration of EDA1, however, showed normal tail skin proliferation, no signs of kinking and, interestingly, a normalized vertebral bone density. Thus, our data prove the causal relationship between EDA1 deficiency and kinky tails and indicate that hair follicles are required for murine tail skin to grow fast enough. Disturbed bone development appears to be partially pre-determined in utero and can be counteracted by timely EDA1 replacement, pointing to a role of EDA1 also in osteogenesis.
Keywords: NF-κB; bone; development; ectodermal dysplasia; ectodysplasin A1; fetal therapy.
Copyright © 2021 Kossel, Wahlbuhl, Schuepbach-Mallepell, Park, Kowalczyk-Quintas, Seeling, von der Mark, Schneider and Schneider.
Conflict of interest statement
PS, CK-Q, and HS are inventors on patents relevant to this publication. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources

