Characterization of tumor-associated MUC1 and its glycans expressed in mucoepidermoid carcinoma
- PMID: 34457057
- PMCID: PMC8358622
- DOI: 10.3892/ol.2021.12963
Characterization of tumor-associated MUC1 and its glycans expressed in mucoepidermoid carcinoma
Abstract
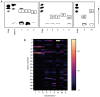
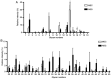
Mucoepidermoid carcinoma (MEC) is one of the most frequently misdiagnosed tumors. Glycans are modulated by malignant transformation. Mucin 1 (MUC1) is a mucin whose expression is upregulated in various tumors, including MEC, and it has previously been investigated as a diagnostic and prognostic tumor marker. The present study aimed to reveal the differences in the mucin glycans between MEC and normal salivary glands (NSGs) to discover novel diagnostic markers. Soluble fractions of salivary gland homogenate prepared from three MEC salivary glands and 7 NSGs were evaluated. Mucins in MEC and NSGs were separated using supported molecular matrix electrophoresis, and stained with Alcian blue and monoclonal antibodies. The glycans of the separated mucins were analyzed by mass spectrometry. MUC1 was found in MEC but not in NSGs, and almost all glycans of MUC1 in MEC were sialylated, whereas the glycans of mucins in NSGs were less sialylated. The core 2 type glycans, (Hex)2(HexNAc)2(NeuAc)1 and (Hex)2(HexNAc)2(NeuAc)2, were found to be significantly abundant glycans of MUC1 in MEC. MEC markedly produced MUC1 modified with sialylated core 2 glycans. These data were obtained from the soluble fractions of salivary gland homogenates. These findings provide a basis for the utilization of MUC1 as a serum diagnostic marker for the preoperative diagnosis of MEC.
Keywords: O-glycan; mucin; mucin 1; mucoepidermoid carcinoma; salivary gland.
Copyright: © Isaka et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Expression and localisation of MUC1 modified with sialylated core-2 O-glycans in mucoepidermoid carcinoma.Sci Rep. 2023 Apr 8;13(1):5752. doi: 10.1038/s41598-023-32597-2. Sci Rep. 2023. PMID: 37031283 Free PMC article.
-
MUC1, MUC2, MUC4, and MUC5AC expression in salivary gland mucoepidermoid carcinoma: diagnostic and prognostic implications.Am J Surg Pathol. 2005 Jul;29(7):881-9. doi: 10.1097/01.pas.0000159103.95360.e8. Am J Surg Pathol. 2005. PMID: 15958852
-
Mucoepidermoid carcinoma-associated expression of MUC5AC, MUC5B and mucin-type carbohydrate antigen sialyl-Tn in the parotid gland.Arch Oral Biol. 2017 Oct;82:121-126. doi: 10.1016/j.archoralbio.2017.06.010. Epub 2017 Jun 13. Arch Oral Biol. 2017. PMID: 28628803
-
Diverse glycosylation of MUC1 and MUC2: potential significance in tumor immunity.J Biochem. 1999 Dec;126(6):975-85. doi: 10.1093/oxfordjournals.jbchem.a022565. J Biochem. 1999. PMID: 10578046 Review.
-
Expression of mucin antigens in human cancers and its relationship with malignancy potential.Pathol Int. 1997 Dec;47(12):813-30. doi: 10.1111/j.1440-1827.1997.tb03713.x. Pathol Int. 1997. PMID: 9503463 Review.
Cited by
-
Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: An update for 2021-2022.Mass Spectrom Rev. 2025 May-Jun;44(3):213-453. doi: 10.1002/mas.21873. Epub 2024 Jun 24. Mass Spectrom Rev. 2025. PMID: 38925550 Free PMC article. Review.
-
Expression and localisation of MUC1 modified with sialylated core-2 O-glycans in mucoepidermoid carcinoma.Sci Rep. 2023 Apr 8;13(1):5752. doi: 10.1038/s41598-023-32597-2. Sci Rep. 2023. PMID: 37031283 Free PMC article.
-
Preparation of Soluble Mucin Solutions from the Salivary Glands.Methods Mol Biol. 2024;2763:45-50. doi: 10.1007/978-1-0716-3670-1_3. Methods Mol Biol. 2024. PMID: 38347398
References
-
- EI-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, editors. 4th edition. IARC Press; Lyon: 2017. WHO classification of head and neck tumors.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
