Role of JNK activation in paclitaxel-induced apoptosis in human head and neck squamous cell carcinoma
- PMID: 34457060
- PMCID: PMC8358625
- DOI: 10.3892/ol.2021.12966
Role of JNK activation in paclitaxel-induced apoptosis in human head and neck squamous cell carcinoma
Abstract
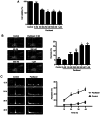
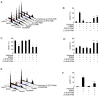
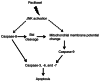
It has been reported that paclitaxel activates cell cycle arrest and increases caspase protein expression to induce apoptosis in head and neck squamous cell carcinoma (HNSCC) cell lines. However, the potential signaling pathway regulating this apoptotic phenomenon remains unclear. The present study used OEC-M1 cells to investigate the underlying molecular mechanism of paclitaxel-induced apoptosis. Following treatment with paclitaxel, cell viability was assessed via the MTT assay. Necrosis, apoptosis, cell cycle and mitochondrial membrane potential (∆Ψm) were analyzed via flow cytometric analyses, respectively. Western blot analysis was performed to detect the expression levels of proteins associated with the MAPK and caspase signaling pathways. The results demonstrated that low-dose paclitaxel (50 nM) induced apoptosis but not necrosis in HNSCC cells. In addition, paclitaxel activated the c-Jun N-terminal kinase (JNK), but not extracellular signal-regulated kinase or p38 mitogen-activated protein kinase. The paclitaxel-activated JNK contributed to paclitaxel-induced apoptosis, activation of caspase-3, -6, -7, -8 and -9, and reduction of ∆Ψm. In addition, caspase-8 and -9 inhibitors, respectively, significantly decreased paclitaxel-induced apoptosis. Notably, Bid was truncated following treatment with paclitaxel. Taken together, the results of the present study suggest that paclitaxel-activated JNK is required for caspase activation and loss of ∆Ψm, which results in apoptosis of HNSCC cells. These results may provide mechanistic basis for designing more effective paclitaxel-combining regimens to treat HNSCC.
Keywords: HNSCC; JNK; apoptosis; caspase; paclitaxel.
Copyright: © Lan et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Paclitaxel induces prolonged activation of the Ras/MEK/ERK pathway independently of activating the programmed cell death machinery.J Biol Chem. 2001 Jun 1;276(22):19555-64. doi: 10.1074/jbc.M011164200. Epub 2001 Mar 5. J Biol Chem. 2001. PMID: 11278851
-
Involvement of Asp-Glu-Val-Asp-directed, caspase-mediated mitogen-activated protein kinase kinase 1 Cleavage, c-Jun N-terminal kinase activation, and subsequent Bcl-2 phosphorylation for paclitaxel-induced apoptosis in HL-60 cells.Mol Pharmacol. 2001 Feb;59(2):254-62. doi: 10.1124/mol.59.2.254. Mol Pharmacol. 2001. PMID: 11160861
-
Inhibition of extracellular signal-regulated kinase (ERK) mediates cell cycle phase independent apoptosis in vinblastine-treated ML-1 cells.Cancer Res. 2001 Feb 15;61(4):1533-40. Cancer Res. 2001. PMID: 11245462
-
Cordycepin Induces Apoptosis through JNK-Mediated Caspase Activation in Human OEC-M1 Oral Cancer Cells.Evid Based Complement Alternat Med. 2022 Oct 3;2022:1842363. doi: 10.1155/2022/1842363. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 38023774 Free PMC article.
-
[The role of mitogen-activated protein kinase cascades in inhibition of proliferation in human prostate carcinoma cells by raloxifene: an in vitro experiment].Zhonghua Yi Xue Za Zhi. 2008 Jan 22;88(4):271-5. Zhonghua Yi Xue Za Zhi. 2008. PMID: 18361842 Chinese.
Cited by
-
Microtubule Targeting Agents in Disease: Classic Drugs, Novel Roles.Cancers (Basel). 2021 Nov 12;13(22):5650. doi: 10.3390/cancers13225650. Cancers (Basel). 2021. PMID: 34830812 Free PMC article. Review.
-
Role of Cannabidiol and Tetrahydrocannabivarin on Paclitaxel-induced neuropathic pain in rodents.Int Immunopharmacol. 2022 Jun;107:108693. doi: 10.1016/j.intimp.2022.108693. Epub 2022 Mar 15. Int Immunopharmacol. 2022. PMID: 35303507 Free PMC article.
-
Arrest and Attack: Microtubule-Targeting Agents and Oncolytic Viruses Employ Complementary Mechanisms to Enhance Anti-Tumor Therapy Efficacy.Genes (Basel). 2024 Sep 11;15(9):1193. doi: 10.3390/genes15091193. Genes (Basel). 2024. PMID: 39336785 Free PMC article. Review.
-
Limocitrin induced cellular death through ERK pathways in human oral squamous cell cancer.Sci Rep. 2025 May 22;15(1):17788. doi: 10.1038/s41598-025-02178-6. Sci Rep. 2025. PMID: 40404781 Free PMC article.
-
Impact of Complex Apoptotic Signaling Pathways on Cancer Cell Sensitivity to Therapy.Cancers (Basel). 2024 Feb 28;16(5):984. doi: 10.3390/cancers16050984. Cancers (Basel). 2024. PMID: 38473345 Free PMC article. Review.
References
-
- Psyrri A, Kwong M, DiStasio S, Lekakis L, Kassar M, Sasaki C, Wilson LD, Haffty BG, Son YH, Ross DA, et al. Cisplatin, fluorouracil, and leucovorin induction chemotherapy followed by concurrent cisplatin chemoradiotherapy for organ preservation and cure in patients with advanced head and neck cancer: Long-term follow-up. J Clin Oncol. 2004;22:3061–3069. doi: 10.1200/JCO.2004.01.108. - DOI - PubMed
-
- Adamo V, Ferraro G, Pergolizzi S, Sergi C, Laudani A, Settineri N, Alafaci E, Scimone A, Spano F, Spitaleri G. Paclitaxel and cisplatin in patients with recurrent and metastatic head and neck squamous cell carcinoma. Oral Oncol. 2004;40:525–531. doi: 10.1016/j.oraloncology.2003.10.010. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
