A study on selective transformation of norbornadiene into fluorinated cyclopentane-fused isoxazolines
- PMID: 34457076
- PMCID: PMC8372314
- DOI: 10.3762/bjoc.17.132
A study on selective transformation of norbornadiene into fluorinated cyclopentane-fused isoxazolines
Abstract
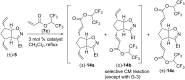
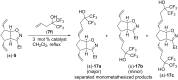
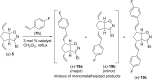
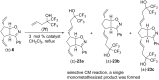
This work presents an examination of the selective functionalization of norbornadiene through nitrile oxide 1,3-dipolar cycloaddition/ring-opening metathesis (ROM)/cross-metathesis (CM) protocols. Functionalization of commercially available norbornadiene provided novel bicyclic scaffolds with multiple stereogenic centers. The synthesis involved selective cycloadditions, with subsequent ROM of the formed cycloalkene-fused isoxazoline scaffolds and selective CM by chemodifferentiation of the olefin bonds of the resulting alkenylated derivatives. Various experimental conditions were applied for the CM transformations with the goal of exploring substrate and steric effects, catalyst influence and chemodifferentiation of the olefin bonds furnishing the corresponding functionalized, fluorine-containing isoxazoline derivatives.
Keywords: functionalization; metathesis; nitrile oxide; organofluorine chemistry; selectivity.
Copyright © 2021, Benke et al.
Figures


















Similar articles
-
Diversity-oriented Functionalization of Cyclodienes Through Selective Cycloaddition/Ring-opening/Cross-metathesis Protocols; Transformation of a "Flatland" into Three-dimensional Scaffolds With Stereo- and Regiocontrol.Chem Rec. 2020 Oct;20(10):1129-1141. doi: 10.1002/tcr.202000070. Epub 2020 Jul 28. Chem Rec. 2020. PMID: 32720742 Review.
-
Ring-opening metathesis of some strained bicyclic systems; stereocontrolled access to diolefinated saturated heterocycles with multiple stereogenic centers.Beilstein J Org Chem. 2018 Oct 24;14:2698-2707. doi: 10.3762/bjoc.14.247. eCollection 2018. Beilstein J Org Chem. 2018. PMID: 30410631 Free PMC article.
-
Nitrile Oxide, Alkenes, Dipolar Cycloaddition, Isomerization and Metathesis Involved in the Syntheses of 2-Isoxazolines.Molecules. 2023 Mar 10;28(6):2547. doi: 10.3390/molecules28062547. Molecules. 2023. PMID: 36985520 Free PMC article. Review.
-
Selective Transformation of Norbornadiene into Functionalized Azaheterocycles and β-Amino Esters with Stereo- and Regiocontrol.Chem Asian J. 2021 Dec 1;16(23):3873-3881. doi: 10.1002/asia.202100956. Epub 2021 Sep 14. Chem Asian J. 2021. PMID: 34498420
-
Synthesis and biological evaluation of new bicyclic fluorinated uracils through ring-closing metathesis.J Org Chem. 2006 May 12;71(10):4010-3. doi: 10.1021/jo0601765. J Org Chem. 2006. PMID: 16674087
References
LinkOut - more resources
Full Text Sources
