Moving Towards Targeted Therapies for Triple-Negative Breast Cancer
- PMID: 34457184
- PMCID: PMC8386298
- DOI: 10.1007/s12609-021-00416-0
Moving Towards Targeted Therapies for Triple-Negative Breast Cancer
Abstract
Purpose of review: In this review, we discuss targets of interest in Triple-negative breast cancer (TNBC), approved targeted agents and the results of the clinical trials that led to their approval. Additionally, we review ongoing clinical trials evaluating the use of novel targeted agents in the treatment of TNBC.
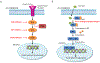
Recent findings: TNBC accounts for 15-20% of all breast cancer cases and is associated with worse clinical outcomes. Patients have a higher risk of metastatic recurrence and inferior overall survival compared to other breast cancer subtypes. Cytotoxic chemotherapy has historically been the mainstay of treatment for TNBC. In recent years, we have seen a surge in clinical trials investigating the use of targeted agents in TNBC and now have approval for targeted therapies in select patients. Inhibitors of PARP (olaparib and talazoparib), PD-L1 (atezolizumab) and an antibody drug conjugate targeting Trop-2 (sacituzumab govitecan-hziy) are now approved for the use in select groups of patients with TNBC.
Summary: Various novel targeted agents as monotherapy, dual targeted combinations, and chemotherapy combinations are currently under investigation. The results are promising and may significantly improve patient outcomes in TNBC.
Keywords: immunotherapy; novel agents; targeted therapies; triple-negative breast cancer.
Conflict of interest statement
Conflict of Interest Jodi A. Kagihara, Elena Shagisultanova, Anosheh Afghahi, and Jennifer R. Diamond declare that they have no conflict of interest
Figures
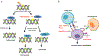

References
-
- Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al.American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784–95. doi: 10.1200/jco.2009.25.6529. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
