Modification of Proteins Using Olefin Metathesis
- PMID: 34457313
- PMCID: PMC8388616
- DOI: 10.1039/c9qm00494g
Modification of Proteins Using Olefin Metathesis
Abstract
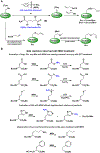
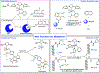
Olefin metathesis has revolutionized synthetic approaches to carbon-carbon bond formation. With a rich history beginning in industrial settings through its advancement in academic laboratories leading to new and highly active metathesis catalysts, olefin metathesis has found use in the generation of complex natural products, the cyclization of bioactive materials, and in the polymerization of new and unique polymer architectures. Throughout this review, we will trace the deployment of olefin metathesis-based strategies for the modification of proteins, a process which has been facilitated by the extensive development of stable, isolable, and highly active transition-metal-based metathesis catalysts. We first begin by summarizing early works which detail peptide modification strategies that played a vital role in identifying stable metathesis catalysts. We then delve into protein modification using cross metathesis and finish with recent work on the generation of protein-polymer conjugates through ring-opening metathesis polymerization.
Conflict of interest statement
Conflicts of interest There are no conflicts to declare.
Figures
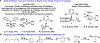





Similar articles
-
Development and application of FI catalysts for olefin polymerization: unique catalysis and distinctive polymer formation.Acc Chem Res. 2009 Oct 20;42(10):1532-44. doi: 10.1021/ar900030a. Acc Chem Res. 2009. PMID: 19588950
-
Degradable polymers via olefin metathesis polymerization.Prog Polym Sci. 2021 Sep;120:101427. doi: 10.1016/j.progpolymsci.2021.101427. Epub 2021 Jun 7. Prog Polym Sci. 2021. PMID: 38666185 Free PMC article.
-
Supported Catalysts Useful in Ring-Closing Metathesis, Cross Metathesis, and Ring-Opening Metathesis Polymerization.Polymers (Basel). 2016 Apr 12;8(4):140. doi: 10.3390/polym8040140. Polymers (Basel). 2016. PMID: 30979231 Free PMC article. Review.
-
Alkane metathesis by tandem alkane-dehydrogenation-olefin-metathesis catalysis and related chemistry.Acc Chem Res. 2012 Jun 19;45(6):947-58. doi: 10.1021/ar3000713. Epub 2012 May 15. Acc Chem Res. 2012. PMID: 22584036
-
Microwave-assisted Olefin Metathesis as Pivotal Step in the Synthesis of Bioactive Compounds.Curr Med Chem. 2017;24(41):4538-4578. doi: 10.2174/0929867324666170314122820. Curr Med Chem. 2017. PMID: 28292236 Review.
Cited by
-
An overview of chemo- and site-selectivity aspects in the chemical conjugation of proteins.R Soc Open Sci. 2022 Jan 26;9(1):211563. doi: 10.1098/rsos.211563. eCollection 2022 Jan. R Soc Open Sci. 2022. PMID: 35116160 Free PMC article.
-
Water-Accelerated Decomposition of Olefin Metathesis Catalysts.ACS Catal. 2023 Jan 3;13(2):1097-1102. doi: 10.1021/acscatal.2c05573. eCollection 2023 Jan 20. ACS Catal. 2023. PMID: 36714054 Free PMC article.
-
Optimization of Ring-Opening Metathesis Polymerization (ROMP) under Physiologically Relevant Conditions.Polym Chem. 2020 Jul 21;11(27):4492-4499. doi: 10.1039/d0py00716a. Epub 2020 Jun 18. Polym Chem. 2020. PMID: 33796158 Free PMC article.
-
Anionic Olefin Metathesis Catalysts Enable Modification of Unprotected Biomolecules in Water.ACS Catal. 2024 Jul 11;14(15):11147-11152. doi: 10.1021/acscatal.4c02811. eCollection 2024 Aug 2. ACS Catal. 2024. PMID: 39114091 Free PMC article.
-
Modular and diverse synthesis of amino acids via asymmetric decarboxylative protonation of aminomalonic acids.Nat Chem. 2023 Dec;15(12):1672-1682. doi: 10.1038/s41557-023-01362-3. Epub 2023 Nov 16. Nat Chem. 2023. PMID: 37973941
References
-
- Trnka TM and Grubbs RH, Acc. Chem. Res, 2001, 34, 18–29. - PubMed
-
- Calderon N, Acc. Chem. Res, 1972, 5, 127–132.
-
- Katz TJ, Adv. Organomet. Chem, 1977, 16, 283–317.
-
- Grubbs RH, in Progress in Inorganic Chemistry, ed. by SJ Lippard, John Wiley & Sons, Inc., 1978, vol. 24, ch. 1, pp. 1–50.
-
- Bielawski CW and Grubbs RH, Progr. Polym. Sci, 2007, 32, 1–29.
Grants and funding
LinkOut - more resources
Full Text Sources
