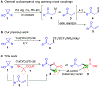
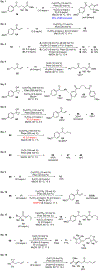
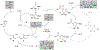Catalytic Cyclopropanol Ring Opening for Divergent Syntheses of γ-Butyrolactones and δ-Ketoesters Containing All-Carbon Quaternary Centers
- PMID: 34457375
- PMCID: PMC8389773
- DOI: 10.1021/acscatal.8b00711
Catalytic Cyclopropanol Ring Opening for Divergent Syntheses of γ-Butyrolactones and δ-Ketoesters Containing All-Carbon Quaternary Centers
Abstract
Catalytic ring opening cross coupling reactions of strained cyclopropanols have been useful for the syntheses of various β-substituted carbonyl products. Among these ring opening cross coupling reactions, the formation of α,β-unsaturated enone byproducts often competes with the desired cross coupling processes and has been a challenging synthetic problem to be addressed. Herein, we describe our efforts in developing divergent syntheses of a wide range of γ-butyrolactones and δ-ketoesters containing all-carbon quaternary centers via copper-catalyzed cyclopropanol ring opening cross couplings with 2-bromo-2,2-dialkyl esters. Our mechanistic studies reveal that unlike the previously reported cases, the formation of α,β-unsaturated enone intermediates is actually essential for the γ-butyrolactone synthesis and also contributes to the formation of the δ-ketoester product. The γ-butyrolactone synthesis is proposed to go through an intermolecular radical conjugate addition to the in situ generated α,β-unsaturated enone followed by an intramolecular radical cyclization to the ester carbonyl double bond. The reactions are effective to build all-carbon quaternary centers and have broad substrate scope.
Keywords: copper catalysis; cyclopropanol; quaternary carbon; ring opening; α,β-unsaturated enone; γ-butyrolactone; δ-ketoester.
Figures
References
-
- For selected examples:Iwasawa N; Matsuo T; Iwamoto M; Ikeno T Rearrangement of 1-(1-Alkynyl)cyclopropanols to 2-Cyclopentenones via Their Hexacarbonyldicobalt Complexes. A New Use of Alkyne–Co2(CO)6 Complexes in Organic Synthesis. J. Am. Chem. Soc, 1998, 120, 3903–3914.
- Markham JP; Staben ST; Toste FD Gold(I)-Catalyzed Ring Expansion of Cyclopropanols and Cyclobutanols. J. Am. Chem. Soc, 2005, 127, 9708–9709. - PubMed
- Trost BM; Xie J; Maulide N Stereoselective, Dual-Mode Ruthenium-Catalyzed Ring Expansion of Alkynylcyclopropanols. J. Am. Chem. Soc 2008, 130, 17258–17259. - PMC - PubMed
- Kleinbeck F; Toste FD Gold(I)-Catalyzed Enantioselective Ring Expansion of Allenylcyclopropanols. J. Am. Chem. Soc 2009, 131, 9178–9719. - PMC - PubMed
- Shu X-Z; Zhang M; He Y; Frei H; Toste FD Dual Visible Light Photoredox and Gold-Catalyzed Arylative Ring Expansion. J. Am. Chem. Soc, 2014, 136, 5844–5847. - PMC - PubMed
- Sethofer SG; Staben ST; Hung OY; Toste FD Au(I)-Catalyzed Ring Expanding Cycloisomerizations: Total Synthesis of Ventricosene. Org. Lett 2008, 10, 4315–4318. - PMC - PubMed
- Tumma N; Gyanchander E; Cha JK Cu-Mediated Rearrangements of Allenylcyclopropanols to Cyclopentenones: Two Divergent Pathways. J. Org. Chem 2017, 82, 4379–4385. - PubMed
- Jiao L; Yuan C; Yu Z-X Tandem Rh(I)-Catalyzed [(5+2)+1] Cycloaddition/Aldol Reaction for the Construction of Linear Triquinane Skeleton: Total Syntheses of (±)-Hirsutene and (±)-1-Desoxyhypnophilin. J. Am. Chem. Soc, 2008, 130, 4421–4430. - PubMed
- Kingsbury JS; Corey EJ Enantioselective Total Synthesis of Isoedunol and β-Araneosene Featuring Unconventional Strategy and Methodology. J. Am. Chem. Soc, 2005, 127, 13813–13815. - PubMed
- Kim K; Cha JK Total Synthesis of Cyathin A3 and Cyathin B2 Angew. Chem., Int. Ed 2009, 48, 5334–5336. - PMC - PubMed
-
- For reviews, see:Gibson DH; DePuy CH Cyclopropanol chemistry. Chem. Rev, 1974, 74, 605–623.
- Kulinkovich OG The Chemistry of Cyclopropanols. Chem. Rev, 2003, 103, 2597–2632. - PubMed
- Mack DJ; Njardarson JT Recent Advances in the Metal-Catalyzed Ring Expansions of Three- and Four-Membered Rings. ACS Catal., 2013, 3, 272–286.
- Nikolaev A; Orellana A Transition-Metal-Catalyzed C─C and C─X Bond-Forming Reactions Using Cyclopropanols. Synthesis 2016, 48, 1741–1768.
- Fumagalli G; Stanton S; Bower JF Recent Methodologies That Exploit C─C Single-Bond Cleavage of Strained Ring Systems by Transition Metal Complexes. Chem. Rev, 2017, 117, 9404–9432. - PubMed
- Ebner C; Carreira EM Cyclopropanation Strategies in Recent Total Syntheses. Chem. Rev, 2017, 117, 11651–11679. - PubMed
-
- For selected examples:Aoki S; Fujimura T; Nakamura E; Kuwajima I Palladium-Catalyzed Arylation of Siloxycyclopropanes with Aryl Triflates. Carbon Chain Elongation via Catalytic Carbon-Carbon Bond Cleavage. J. Am. Chem. Soc 1988, 110, 3296–3298.
- Fujimura T; Aoki S; Nakamura E Synthesis of 1,4-Keto Esters and 1,4-Diketones via Palladium-Catalyzed Acylation of Siloxycyclopropanes. Synthetic and Mechanistic Studies. J. Org. Chem 1991, 56, 2809–2821.
- Aoki S; Nakamura E Synthesis of 1,4-Dicarbonyl Compounds by Palladium-Catalyzed Carbonylative Arylation of Siloxycyclopropanes. Synlett 1990, 741–742.
- Kang S-K; Yamaguchi T; Ho P-S; Kim W-Y; Yoon S-K Palladium-Catalyzed Coupling and Carbonylative Coupling of Silyloxy Compounds with Hypervalent Iodonium Salts. Tetrahedron Lett. 1997, 38, 1947–1950.
- Rosa D; Orellana A Palladium-Catalyzed Cross-Coupling of Cyclopropanols with Aryl Halides Under Mild Conditions. Org. Lett 2011, 13, 110–113. - PubMed
- Rosa D; Orellana A Synthesis of α-indanones via Intramolecular Direct Arylation with Cyclopropanol-Derived Homoenolates. Chem. Commun 2012, 48, 1922–1924. - PubMed
- Parida BB; Das PP; Niocel M; Cha JK C-Acylation of Cyclopropanols: Preparation of Functionalized 1,4-Diketones. Org. Lett 2013, 15, 1780–1783. - PubMed
- Cheng K; Walsh PJ Arylation of Aldehyde Homoenolates with Aryl Bromides. Org. Lett 2013, 15, 2298–2301. - PubMed
- Das PP; Belmore K; Cha JK SN2’ Alkylation of Cyclopropanols via Homoenolates. Angew. Chem., Int. Ed 2012, 51, 9517–9520. - PubMed
- Rao NN; Parida BB; Cha JK Cross-Coupling of Cyclopropanols: Concise Syntheses of Indolizidine 223AB and Congeners. Org. Lett 2014, 16, 6208–6211. - PubMed
- Rao NN; Cha JK Concise Synthesis of Alkaloid (–)−205B. J. Am. Chem. Soc 2015, 137, 2243–2246. - PubMed
- Murali RVNS; Rao NN; Cha JK C-Alkynylation of Cyclopropanols. Org. Lett 2015, 17, 3854–3856. - PubMed
- Gyanchander E; Ydhyam S; Tumma N; Belmore K; Cha JK Mechanism of Ru(II)-Catalyzed Rearrangements of Allenyl- and Alkynylcyclopropanols to Cyclopentenones. Org. Lett 2016, 18, 6098–6101. - PubMed
- Kananovich DG; Konik YA; Zubrytski DM; Järving I; Lopp M Simple Access to β-Trifluoromethyl-Substituted Ketones via Copper-Catalyzed Ring-Opening TrifluoroMethylation of Substituted Cyclopropanols. Chem. Commun 2015, 51, 8349–8352. - PubMed
- Jia K; Zhang F; Huang H; Chen Y Visible-Light-Induced Alkoxyl Radical Generation Enables Selective C(sp3)─C(sp3) Bond Cleavage and Functionalizations. J. Am. Chem. Soc 2016, 138, 1514–1517. - PubMed
- Zhang H; Wu G; Yi H; Sun T; Wang B; Zhang Y; Dong G; Wang J Copper(I)-Catalyzed Chemoselective Coupling of Cyclopropanols with Diazoesters: Ring-Opening C─C Bond Formations. Angew. Chem. Int. Ed 2017, 56, 3945–3950. - PubMed
- Zhou X; Yu S; Kong L; Li X Rhodium(III)-Catalyzed Coupling of Arenes with Cyclopropanols via C─H Activation and Ring Opening. ACS Catal., 2016, 6, 647–651. - PubMed
-
- Jiao J; Nguyen LX; Patterson DR; Flowers RA II. An Efficient and General Approach to β-Functionalized Ketones. Org. Lett 2007, 9, 1323–1326. - PMC - PubMed
- Zhao H; Fan X; Yu J; Zhu C Silver-Catalyzed Ring-Opening Strategy for the Synthesis of β- and γ-Fluorinated Ketones. J. Am. Chem. Soc 2015, 137, 3490–3493. - PubMed
- Bloom S; Bume DD; Pitts CR; Lectka T Site-Selective Approach to β-Fluorination: Photocatalyzed Ring Opening of Cyclopropanols. Chem. - Eur. J 2015, 13, 8060–8063. - PubMed
- Ren S; Feng C; Loh T-P Iron- or Silver-Catalyzed Oxidative Fluorination of Cyclopropanols for the Synthesis of β-Fluoroketones. Org. Biomol. Chem 2015, 13, 5105–5109. - PubMed
- Huang F-Q; Xie J; Sun J-G; Wang Y-W; Dong X; Qi L-W; Zhang B Regioselective Synthesis of Carbonyl-Containing Alkyl Chlorides via Silver-Catalyzed Ring-Opening Chlorination of Cycloalkanols. Org. Lett 2016, 18, 684–687. - PubMed
- Bume DD; Pitts CR; Lectka T Tandem C─C Bond Cleavage of -Cyclopropanols and Oxidative Aromatization by Manganese(IV) Oxide in a Direct C─H to C─C Functionalization of Heteroaromatics. Eur. J. Org. Chem 2016, 26–30.
- Wang S; Guo L-N; Wang H; Duan X-H Alkynylation of Tertiary Cycloalkanols via Radical C─C Bond Cleavage: A Route to Distal Alkynylated Ketones. Org. Lett 2015, 17, 4798–4801. - PubMed
- Wang Y-F; Chiba S Mn(III)-Mediated Reactions of Cyclopropanols with Vinyl Azides: Synthesis of Pyridine and 2-Azabicyclo[3.3.1]non-2-en-1-ol Derivatives. J. Am. Chem. Soc 2009, 131, 12570–12572. - PubMed
- Wang Y-F; Toh KK; Ng EPJ; Chiba S Mn(III)-Mediated Formal [3+3]-Annulation of Vinyl Azides and Cyclopropanols: A Divergent Synthesis of Azaheterocycles. J. Am. Chem. Soc 2011, 133, 6411–6421. - PubMed
- Iwasawa N; Hayakawa S; Funahashi M; Isobe K; Narasaka K Generation of β-Carbonyl Radicals from Cyclopropanol Derivatives by the Oxidation with Manganese(III) 2-Pyridinecarboxylate and Their Reactions with Electron-Rich and -Deficient Olefins. Bull. Chem. Soc. Jpn 1993, 66, 819–827.
- Chiba S; Cao Z; El Bialy SAA; Narasaka K Generation of β-Keto Radicals from Cyclopropanols Catalyzed by AgNO3. Chem. Lett 2006, 35, 18–19.
- Chiba S; Kitamura M; Narasaka K Synthesis of (–)-Sordarin. J. Am. Chem. Soc 2006, 128, 6931–6937. - PubMed
- Ilangovan A; Saravanakumar S; Malayappasamy S γ-Carbonyl Quinones: Radical Strategy for the Synthesis of Evelynin and Its Analogues by C─H Activation of Quinones Using Cyclopropanols. Org. Lett 2013, 15, 4968–4971. - PubMed
- Deng Y; Kauser NI; Islam SM; Mohr JT AgII-Mediated Synthesis of β-Fluoroketones by Oxidative Cyclopropanol Opening. Eur. J. Org. Chem 2017, 5872–5879.
- Wang CY; Song R-J; Xie Y-X; Li J-H Silver-Promoted Oxidative Ring Opening/Alkynylation of Cyclopropanols: Facile Synthesis of 4-Yn-1-ones. Synthesis 2016, 48, 223–230.
- Lu S-C; Li H-S; Xu S; Duan G-Y Silver-Catalyzed C2-Selective Direct Alkylation of Heteroarenes with Tertiary Cycloalkanols. Org. Biomol. Chem 2017, 15, 324–327. - PubMed
- Ishida N; Okumura S; Nakanishi Y; Murakami M Ring-opening Fluorination of Cyclobutanols and Cyclopropanols Catalyzed by Silver. Chem. Lett 2015, 44, 821–823.
- Keaton KA; Phillips AJ A Cyclopropanol-Based Strategy for Subunit Coupling: Total Synthesis of (+)-Spirolaxine Methyl Ether. Org. Lett 2007, 9, 2717–2719. - PubMed
-
- Park S-B; Cha JK Palladium-Mediated Ring Opening of Hydroxycyclopropanes. Org. Lett 2000, 2, 147–149. - PubMed
- Okumoto H; Jinnai T; Shimizu H; Harada Y; Mishima H; Suzuki A Pd-Catalyzed Ring Opening of Cyclopropanols. Synlett 2000, 629–630.
Grants and funding
LinkOut - more resources
Full Text Sources