Towards personalized and versatile monitoring of temperature fields within heterogeneous tissues during laser therapies
- PMID: 34457430
- PMCID: PMC8367272
- DOI: 10.1364/BOE.428028
Towards personalized and versatile monitoring of temperature fields within heterogeneous tissues during laser therapies
Abstract
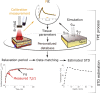
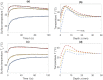
Advancements in medical laser technology have paved the way for its widespread acceptance in a variety of treatments and procedures. Selectively targeting particular tissue structures with minimally invasive procedures limits the damage to surrounding tissue and allows for reduced post-procedural downtime. In many treatments that are hyperthermia-based, the efficiency depends on the achieved temperature within the targeted tissues. Current approaches for monitoring subdermal temperature distributions are either invasive, complex, or offer inadequate spatial resolution. Numerical studies are often therapy-tailored and source tissue parameters from the literature, lacking versatility and a tissue-specific approach. Here, we show a protocol that estimates the temperature distribution within the tissue based on a thermographic recording of its surface temperature evolution. It couples a time-dependent matching algorithm and thermal-diffusion-based model, while recognizing tissue-specific characteristics yielded by a fast calibration process. The protocol was employed during hyperthermic laser treatment performed ex-vivo on a heterogeneous porcine tissue, and in-vivo on a human subject. In both cases the calibrated thermal parameters correlate with the range of values reported by other studies. The matching algorithm sufficiently reproduced the temperature dynamics of heterogeneous tissue. The estimated temperature distributions within ex-vivo tissue were validated by simultaneous reference measurements, and the ones estimated in-vivo reveal a distribution trend that correlates well with similar studies. The presented method is versatile, supported by the protocol for tissue-specific tailoring, and can readily be implemented for temperature monitoring of various hyperthermia-based procedures by means of recording the surface temperature evolution with a miniature thermal camera implemented within a handheld laser scanner or similar.
© 2021 Optical Society of America under the terms of the OSA Open Access Publishing Agreement.
Conflict of interest statement
The authors declare that there are no conflict of interest.
Figures


Similar articles
-
Non-contact monitoring of the depth temperature profile for medical laser scanning technologies.Sci Rep. 2020 Nov 20;10(1):20242. doi: 10.1038/s41598-020-77283-9. Sci Rep. 2020. PMID: 33219279 Free PMC article.
-
Interstitial laser hyperthermia model development for minimally invasive therapy of breast carcinoma.J Am Coll Surg. 1998 Mar;186(3):284-92. doi: 10.1016/s1072-7515(97)00152-x. J Am Coll Surg. 1998. PMID: 9510259
-
Application of Ultrasound Thermal Imaging for Monitoring Laser Ablation in Ex Vivo Cardiac Tissue.Lasers Surg Med. 2020 Mar;52(3):218-227. doi: 10.1002/lsm.23157. Epub 2019 Sep 7. Lasers Surg Med. 2020. PMID: 31493345
-
Techniques for temperature monitoring during laser-induced thermotherapy: an overview.Int J Hyperthermia. 2013 Nov;29(7):609-19. doi: 10.3109/02656736.2013.832411. Epub 2013 Sep 13. Int J Hyperthermia. 2013. PMID: 24032415 Review.
-
Magnetic resonance thermometry and its biological applications - Physical principles and practical considerations.Prog Nucl Magn Reson Spectrosc. 2019 Feb;110:34-61. doi: 10.1016/j.pnmrs.2019.01.003. Epub 2019 Jan 31. Prog Nucl Magn Reson Spectrosc. 2019. PMID: 30803693 Free PMC article. Review.
Cited by
-
Interferometric imaging of thermal expansion for temperature control in retinal laser therapy.Biomed Opt Express. 2022 Jan 13;13(2):728-743. doi: 10.1364/BOE.448803. eCollection 2022 Feb 1. Biomed Opt Express. 2022. PMID: 35284191 Free PMC article.
-
Multiphysical numerical study of photothermal therapy of glioblastoma with photoacoustic temperature monitoring in a mouse head.Biomed Opt Express. 2022 Feb 3;13(3):1202-1223. doi: 10.1364/BOE.444193. eCollection 2022 Mar 1. Biomed Opt Express. 2022. PMID: 35414964 Free PMC article.
References
-
- Nouri K., Lasers in Dermatology and Medicine (Springer Science + Business Media B.V., 2011).
LinkOut - more resources
Full Text Sources
