mRNA-based therapy in a rabbit model of variegate porphyria offers new insights into the pathogenesis of acute attacks
- PMID: 34458006
- PMCID: PMC8368795
- DOI: 10.1016/j.omtn.2021.05.010
mRNA-based therapy in a rabbit model of variegate porphyria offers new insights into the pathogenesis of acute attacks
Abstract
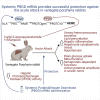
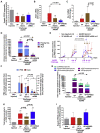
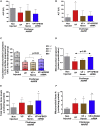
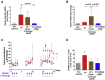
Variegate porphyria (VP) results from haploinsufficiency of protoporphyrinogen oxidase (PPOX), the seventh enzyme in the heme synthesis pathway. There is no VP model that recapitulates the clinical manifestations of acute attacks. Combined administrations of 2-allyl-2-isopropylacetamide and rifampicin in rabbits halved hepatic PPOX activity, resulting in increased accumulation of a potentially neurotoxic heme precursor, lipid peroxidation, inflammation, and hepatocyte cytoplasmic stress. Rabbits also showed hypertension, motor impairment, reduced activity of critical mitochondrial hemoprotein functions, and altered glucose homeostasis. Hemin treatment only resulted in a slight drop in heme precursor accumulation but further increased hepatic heme catabolism, inflammation, and cytoplasmic stress. Hemin replenishment did protect against hypertension, but it failed to restore action potentials in the sciatic nerve or glucose homeostasis. Systemic porphobilinogen deaminase (PBGD) mRNA administration increased hepatic PBGD activity, the third enzyme of the pathway, and rapidly normalized serum and urine porphyrin precursor levels. All features studied were improved, including those related to critical hemoprotein functions. In conclusion, the VP model recapitulates the biochemical characteristics and some clinical manifestations associated with severe acute attacks in humans. Systemic PBGD mRNA provided successful protection against the acute attack, indicating that PBGD, and not PPOX, was the critical enzyme for hepatic heme synthesis in VP rabbits.
Keywords: characterization of variegate porphyria rabbits; hepatic heme synthesis; lipid nanoparticles; mRNA delivery; pharmacological model of variegate porphyria; rare metabolic disease.
© 2021 The Author(s).
Conflict of interest statement
L.J. and P.G.V.M. are employees of Moderna Inc., focusing on the development of therapeutic approaches for rare diseases. The remaining authors declare no competing interests.
Figures

Similar articles
-
Systemic messenger RNA replacement therapy is effective in a novel clinically relevant model of acute intermittent porphyria developed in non-human primates.Gut. 2025 Jan 17;74(2):270-283. doi: 10.1136/gutjnl-2024-332619. Gut. 2025. PMID: 39366725 Free PMC article.
-
Variegate Porphyria.2013 Feb 14 [updated 2019 Dec 12]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2013 Feb 14 [updated 2019 Dec 12]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 23409300 Free Books & Documents. Review.
-
Systemic messenger RNA as an etiological treatment for acute intermittent porphyria.Nat Med. 2018 Dec;24(12):1899-1909. doi: 10.1038/s41591-018-0199-z. Epub 2018 Oct 8. Nat Med. 2018. PMID: 30297912
-
Haem Biosynthesis and Antioxidant Enzymes in Circulating Cells of Acute Intermittent Porphyria Patients.PLoS One. 2016 Oct 27;11(10):e0164857. doi: 10.1371/journal.pone.0164857. eCollection 2016. PLoS One. 2016. PMID: 27788171 Free PMC article.
-
Hepatic porphyria: A narrative review.Indian J Gastroenterol. 2016 Nov;35(6):405-418. doi: 10.1007/s12664-016-0698-0. Epub 2016 Oct 31. Indian J Gastroenterol. 2016. PMID: 27796941 Review.
Cited by
-
Generation and characterization of human U-2 OS cell lines with the CRISPR/Cas9-edited protoporphyrinogen oxidase IX gene.Sci Rep. 2022 Oct 12;12(1):17081. doi: 10.1038/s41598-022-21147-x. Sci Rep. 2022. PMID: 36224252 Free PMC article.
-
Cutting-Edge Therapies and Novel Strategies for Acute Intermittent Porphyria: Step-by-Step towards the Solution.Biomedicines. 2022 Mar 11;10(3):648. doi: 10.3390/biomedicines10030648. Biomedicines. 2022. PMID: 35327450 Free PMC article. Review.
-
Systemic messenger RNA replacement therapy is effective in a novel clinically relevant model of acute intermittent porphyria developed in non-human primates.Gut. 2025 Jan 17;74(2):270-283. doi: 10.1136/gutjnl-2024-332619. Gut. 2025. PMID: 39366725 Free PMC article.
-
Recent Insights into the Pathogenesis of Acute Porphyria Attacks and Increasing Hepatic PBGD as an Etiological Treatment.Life (Basel). 2022 Nov 11;12(11):1858. doi: 10.3390/life12111858. Life (Basel). 2022. PMID: 36430993 Free PMC article. Review.
-
Frameworks for transformational breakthroughs in RNA-based medicines.Nat Rev Drug Discov. 2024 Jun;23(6):421-444. doi: 10.1038/s41573-024-00943-2. Epub 2024 May 13. Nat Rev Drug Discov. 2024. PMID: 38740953 Review.
References
-
- Puy H., Gouya L., Deybach J.-C. Porphyrias. Lancet. 2010;375:924–937. - PubMed
-
- Stein P.E., Badminton M.N., Rees D.C. Update review of the acute porphyrias. Br. J. Haematol. 2017;176:527–538. - PubMed
-
- Bissell D.M., Anderson K.E., Bonkovsky H.L. Porphyria. N. Engl. J. Med. 2017;377:862–872. - PubMed
-
- Stölzel U., Doss M.O., Schuppan D. Clinical guide and update on porphyrias. Gastroenterology. 2019;157:365–381.e4. - PubMed
-
- Junkins-Hopkins J.M. Porphyrias. Clin. Pathol. Asp. Ski. Dis. Endocrine, Metab. Nutr. Depos. Dis. 2010;375:83–90.
LinkOut - more resources
Full Text Sources

