Identification and expression analysis of pathogenicity-related genes of Rhizoctonia solani anastomosis groups infecting rice
- PMID: 34458063
- PMCID: PMC8329123
- DOI: 10.1007/s13205-021-02934-1
Identification and expression analysis of pathogenicity-related genes of Rhizoctonia solani anastomosis groups infecting rice
Abstract
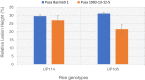
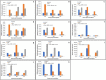
Sheath blight disease caused by Rhizoctonia solani Kuhn (teleomorph; Thanatephorus cucumeris) is a major constraint in rice production. Among the different anastomosis groups (AGs) of Rhizoctonia solani, AG1-IA causes sheath blight of rice, which induce necrotic lesions on leaf sheaths of the infected plants. Several reports contradict the host specificity of anastomosis groups in Rhizoctonia solani. There is lack of information on the pathogenicity genes of these Rhizoctonia solani anastomosis groups during sheath blight infection in rice. In the present study, Rhizoctonia solani isolates collected from diverse rice growing regions of India were screened for anastomosis groups and two groups namely, AG1-IA, AG2-2 were identified. Accordingly, comparative studies were made with AG1-IA (GenBank ID: 16,395) and AG2-2 (GenBank ID: 2,318,768) group sequences, which enabled the identification of specific gene clusters (119 in AG1-IA and 604 in AG2-2) belonging to these groups. Pathogen Host Interaction (PHI) blast with these specific gene clusters could further identify genes involved in host pathogen interaction (38 in AG1_IA and 150 in AG2-2), which were shortlisted for qRT-PCR validation based on qcov cutoff values representing different phenotypic categories of PHI blast. Expression analysis-based validation in sheath blight susceptible (Pusa Basmati 1) and resistant (Pusa 1908-13-12-5) rice genotypes showed that most of the genes expressed significantly higher in the susceptible variety Pusa Basmati 1. The genes like inorganic phosphate transporter (AG1_IPT), Bromodomain containing protein (AG1_BrD), Aldehyde dehydrogenase (AG1_AldD), AMP binding domain (AG1_AMP) and Heme peroxidase (AG1_HmPr) were upregulated in the susceptible genotype, PB 1 at 72hpi compared to Pusa 1908-13-12-5. Among these, inorganic phosphate transporter (AG1_IPT), Bromodomain containing protein (AG1_BrD) and Heme peroxidase (AG1_HmPr) were specific to Rhizoctonia solani AG1-IA. Through the present study, we could demonstrate the AG1-IA-specific interactions of Rhizoctonia solani causing sheath blight disease of rice, which is a step forward in understanding the specificity of Rhizoctonia solani with reference to sheath blight disease of rice.
Supplementary information: The online version contains supplementary material available at 10.1007/s13205-021-02934-1.
Keywords: AG1-IA; AG2-2; Inorganic phosphate transporter; PB 1; Sheath blight.
© King Abdulaziz City for Science and Technology 2021.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest in the publication.
Figures




References
-
- Akhtar J, Jha VK, Lal HC. Occurrence of Banded Leaf and Sheath blight of Maize in Jharkhand with reference to diversity in Rhizoctonia solani. Asian J Agric Sci. 2009;1:32–35.
-
- Anderson NA. The genetics and pathology of Rhizoctonia solani. Annu Rev Phytopathol. 1982;20:329–347. doi: 10.1146/annurev.py.20.090182.001553. - DOI
-
- Bhaktavatsalam G, Satyanarayana K, Reddy APK, John VT. Evaluation for sheath blight resistance in rice. Int Rice Res Newslett. 1978;3:9–10.
-
- Bolkan HA, Ribeiro WRC. Anastomosis groups and pathogenicity of Rhizoctonia solani isolates from Brazil. Plant Dis. 1985;69:599–601. doi: 10.1094/PD-69-599. - DOI
-
- Carling DE. Grouping in Rhizoctonia solani by hyphal anastomosis reactions. In: Sneh B, Jabaji-Hare S, Neate SM, Dijst G, editors. Rhizoctonia species: taxonomy, molecular biology, ecology, pathology and disease control. Netherland: Kluwer Academic Publishers Dordrecht; 1996. pp. 37–47.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

