Novel Bis-Thiazole Derivatives: Synthesis and Potential Cytotoxic Activity Through Apoptosis With Molecular Docking Approaches
- PMID: 34458233
- PMCID: PMC8397418
- DOI: 10.3389/fchem.2021.694870
Novel Bis-Thiazole Derivatives: Synthesis and Potential Cytotoxic Activity Through Apoptosis With Molecular Docking Approaches
Abstract
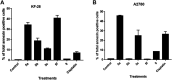
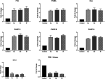
A series of bis-thiazoles 5a-g were synthesized from bis-thiosemicarbazone 3 with hydrazonoyl chlorides 4a-g. Reaction of 3 with two equivalents of α-halocarbonyl compounds 6-8, 10, and 12a-d afforded the corresponding bis-thiazolidines 9, 11, and 13a-d, respectively. Condensation of bis-thiazolidin-4-one 9 with different aromatic aldehydes furnished bis-thiazolidin-4-ones 14a-d. Compounds 5a-g, 9, and 13a,c,d were screened in vitro for their cytotoxic activities in a panel of cancer cell lines. Compounds 5a-c, 5f-g, and 9 exhibited remarkable cytotoxic activities, especially compound 5c with potent IC50 value 0.6 nM (against cervical cancer, Hela cell line) and compound 5f with high IC50 value 6 nM (against ovarian cancer, KF-28 cell line). Compound 5f-induced appreciated apoptotic cell death was measured as 82.76% associated with cell cycle arrest at the G1 phase. The apoptotic pathways activated in KF-28 cells treated with 5a, 5b, and 5f were further investigated. The upregulation of some pro-apoptotic genes, bax and puma, and the downregulation of some anti-apoptotic genes including the Bcl-2 gene were observed, indicating activation of the mitochondrial-dependent apoptosis. Together with the molecular docking studies of compounds 5a and 5b, our data revealed potential Pim-1 kinase inhibition through their high binding affinities indicated by inhibition of phosphorylated C-myc as a downstream target for Pim-1 kinase. Our study introduces a set of bis-thiazoles with potent anti-cancer activities, in vitro.
Keywords: Pim-1 kinase; apoptosis; bis-thiazoles; cytotoxic; docking; hydrazonoyl chlorides.
Copyright © 2021 Dawood, Raslan, Abbas, Mohamed, Abdellattif, Nafie and Hassan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

