3D Organoid Culture From Adult Salivary Gland Tissues as an ex vivo Modeling of Salivary Gland Morphogenesis
- PMID: 34458260
- PMCID: PMC8397473
- DOI: 10.3389/fcell.2021.698292
3D Organoid Culture From Adult Salivary Gland Tissues as an ex vivo Modeling of Salivary Gland Morphogenesis
Abstract
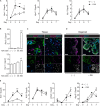
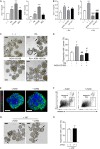
Lumen formation of salivary glands has been investigated using in vivo or ex vivo rudiment culture models. In this study, we used a three-dimensional (3D) salivary gland organoid culture system and demonstrated that lumen formation could be recapitulated in mouse SMG organoids. In our organoid culture system, lumen formation was induced by vasoactive intestinal peptide and accelerated by treatment with RA. Furthermore, lumen formation was observed in branching duct-like structure when cultured in combination of fibroblast growth factors (FGF) in the presence of retinoic acid (RA). We suggest RA signaling-mediated regulation of VIPR1 and KRT7 as the underlying mechanism for lumen formation, rather than apoptosis in the organoid culture system. Collectively, our results support a fundamental role for RA in lumen formation and demonstrate the feasibility of 3D organoid culture as a tool for studying salivary gland morphogenesis.
Keywords: lumen formation; morphogenesis; organoid culture; retinoic acid; salivary gland organoid; stem cells; vasoactive intestinal peptide.
Copyright © 2021 Kim, Yoon, Choi, Kim and Lim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Connexin 43 Is Necessary for Salivary Gland Branching Morphogenesis and FGF10-induced ERK1/2 Phosphorylation.J Biol Chem. 2016 Jan 8;291(2):904-12. doi: 10.1074/jbc.M115.674663. Epub 2015 Nov 12. J Biol Chem. 2016. PMID: 26565022 Free PMC article.
-
3D Cell Culture Models Demonstrate a Role for FGF and WNT Signaling in Regulation of Lung Epithelial Cell Fate and Morphogenesis.Front Cell Dev Biol. 2020 Jul 21;8:574. doi: 10.3389/fcell.2020.00574. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850782 Free PMC article.
-
A Mammary Organoid Model to Study Branching Morphogenesis.Front Physiol. 2022 Mar 16;13:826107. doi: 10.3389/fphys.2022.826107. eCollection 2022. Front Physiol. 2022. PMID: 35399282 Free PMC article.
-
Sox9 function in salivary gland development.J Oral Biosci. 2021 Mar;63(1):8-13. doi: 10.1016/j.job.2021.01.005. Epub 2021 Jan 23. J Oral Biosci. 2021. PMID: 33497841 Review.
-
[Development and future promise of salivary gland organoids and salivary gland tumor organoids].Zhonghua Kou Qiang Yi Xue Za Zhi. 2022 May 9;57(5):535-539. doi: 10.3760/cma.j.cn112144-20220105-00003. Zhonghua Kou Qiang Yi Xue Za Zhi. 2022. PMID: 35484678 Review. Chinese.
Cited by
-
Encapsulation of Primary Salivary Gland Acinar Cell Clusters and Intercalated Ducts (AIDUCs) within Matrix Metalloproteinase (MMP)-Degradable Hydrogels to Maintain Tissue Structure and Function.Adv Healthc Mater. 2022 Apr;11(7):e2101948. doi: 10.1002/adhm.202101948. Epub 2022 Jan 20. Adv Healthc Mater. 2022. PMID: 34994104 Free PMC article.
-
Salivary gland organoid culture maintains distinct glandular properties of murine and human major salivary glands.Nat Commun. 2022 Jun 7;13(1):3291. doi: 10.1038/s41467-022-30934-z. Nat Commun. 2022. PMID: 35672412 Free PMC article.
-
Patient-derived tumor organoids: A preclinical platform for personalized cancer therapy.Transl Oncol. 2025 Jan;51:102226. doi: 10.1016/j.tranon.2024.102226. Epub 2024 Dec 1. Transl Oncol. 2025. PMID: 39622151 Free PMC article. Review.
-
Morphogenesis and development of human telencephalic organoids in the absence and presence of exogenous extracellular matrix.EMBO J. 2023 Nov 15;42(22):e113213. doi: 10.15252/embj.2022113213. Epub 2023 Oct 16. EMBO J. 2023. PMID: 37842725 Free PMC article.
-
Salivary gland stem/progenitor cells: advancing from basic science to clinical applications.Cell Regen. 2025 Jan 24;14(1):4. doi: 10.1186/s13619-025-00221-5. Cell Regen. 2025. PMID: 39856475 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

