Engaging People Who Inject Drugs Living With HIV in Antiretroviral Treatment and Medication for Opioid Use Disorder: Extended Follow-up of HIV Prevention Trials Network (HPTN) 074
- PMID: 34458390
- PMCID: PMC8391093
- DOI: 10.1093/ofid/ofab281
Engaging People Who Inject Drugs Living With HIV in Antiretroviral Treatment and Medication for Opioid Use Disorder: Extended Follow-up of HIV Prevention Trials Network (HPTN) 074
Abstract
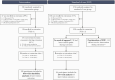
Background: People who inject drugs (PWID) living with HIV experience inadequate access to antiretroviral treatment (ART) and medication for opioid use disorders (MOUD). HPTN 074 showed that an integrated intervention increased ART use and viral suppression over 52 weeks. To examine durability of ART, MOUD, and HIV viral suppression, participants could re-enroll for an extended follow-up period, during which standard-of-care (SOC) participants in need of support were offered the intervention.
Methods: Participants were recruited from Ukraine, Indonesia and Vietnam and randomly allocated 3:1 to SOC or intervention. Eligibility criteria included: HIV-positive; active injection drug use; 18-60 years of age; ≥1 HIV-uninfected injection partner; and viral load ≥1,000 copies/mL. Re-enrollment was offered to all available intervention and SOC arm participants, and SOC participants in need of support (off-ART or off-MOUD) were offered the intervention.
Results: The intervention continuation group re-enrolled 89 participants, and from week 52 to 104, viral suppression (<40 copies/mL) declined from 41% to 29% (estimated 9.4% decrease per year, 95% CI -17.0%; -1.8%). The in need of support group re-enrolled 94 participants and had increased ART (re-enrollment: 55%, week 26: 69%) and MOUD (re-enrollment: 16%, week 26: 25%) use, and viral suppression (re-enrollment: 40%, week 26: 49%).
Conclusions: Viral suppression declined in year 2 for those who initially received the HPTN 074 intervention and improved maintenance support is warranted. Viral suppression and MOUD increased among in need participants who received intervention during the study extension. Continued efforts are needed for widespread implementation of this scalable, integrated intervention.
Keywords: HIV infection; antiretroviral therapy; injection drug use; methadone/therapeutic use; viral load.
© The Author(s) 2021. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures


References
-
- DeHovitz J, Uuskula A, El-Bassel N. The HIV epidemic in Eastern Europe and Central Asia. Curr HIV/AIDS Rep 2014; 11:168–76. - PubMed
-
- Degenhardt L, Charlson F, Stanaway J, et al. . Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C, and hepatitis B: findings from the global burden of disease study 2013. Lancet Infect Dis 2016; 16:1385–98. - PubMed
-
- Stimson G.Drug injecting and HIV infection. Abingdon, UK: Routledge, 1998.
-
- Booth RE, Kwiatkowski CF, Brewster JT, et al. . Predictors of HIV sero-status among drug injectors at three Ukraine sites. AIDS 2006; 20:2217–23. - PubMed

