Rubisco Extraction and Purification from Diatoms
- PMID: 34458500
- PMCID: PMC8376516
- DOI: 10.21769/BioProtoc.2191
Rubisco Extraction and Purification from Diatoms
Abstract
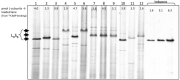
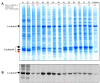
This protocol describes a method to extract ribulose-1,5-bisphosphate carboxylase oxygenase (Rubisco) from diatoms (Bacillariophyta) to determine catalytic performance. This protocol has been adapted from use in cyanobacteria and higher plants (Andrews, 1988; Whitney and Sharwood, 2007). First part (steps A1-A3) of the extraction provides a crude extract of Rubisco that is sufficient for carboxylation assays to measure the Michaelis constant for CO2 (KC) and the catalytic turnover rate ( kcat c ). However, the further purification steps outlined (steps B1-B4) are needed for measurements of Rubisco CO2/O2 Specificity (SC/O, [ Kane et al., 1994 ]).
Keywords: Carbon fixation; Diatoms; Extraction; Phytoplankton; Rubisco.
Copyright © 2017 The Authors; exclusive licensee Bio-protocol LLC.
Figures



References
-
- Andrews T. J.(1988). Catalysis by cyanobacterial ribulose-bisphosphate carboxylase large subunits in the complete absence of small subunits. J Biol Chem 263(25): 12213-12219. - PubMed
-
- Falkowski P. G. and Raven J. A.(2007). Aquatic photosynthesis. Princeton University Press.
-
- Galmes J., Kapralov M. V., Andralojc P. J., Conesa M. A., Keys A. J., Parry M. A. and Flexas J.(2014). Expanding knowledge of the Rubisco kinetics variability in plant species: environmental and evolutionary trends. Plant Cell Environ 37(9): 1989-2001. - PubMed
-
- Kane H. J., Viil J., Entsch B., Paul K., Morell M. K. and Andrews T. J.(1994). An improved method for measuring the CO2/O2 specificity of ribulosebisphosphate carboxylase-oxygenase . Aust J Plant Physiol 21(4): 449-461.
LinkOut - more resources
Full Text Sources
Miscellaneous

