miR-9-5p is involved in the rescue of stress-dependent dendritic shortening of hippocampal pyramidal neurons induced by acute antidepressant treatment with ketamine
- PMID: 34458512
- PMCID: PMC8379501
- DOI: 10.1016/j.ynstr.2021.100381
miR-9-5p is involved in the rescue of stress-dependent dendritic shortening of hippocampal pyramidal neurons induced by acute antidepressant treatment with ketamine
Abstract
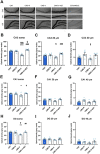
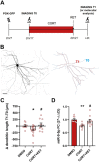
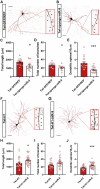
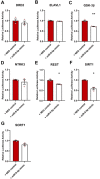
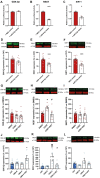
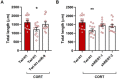
Converging clinical and preclinical evidence demonstrates that depressive phenotypes are associated with synaptic dysfunction and dendritic simplification in cortico-limbic glutamatergic areas. On the other hand, the rapid antidepressant effect of acute ketamine is consistently reported to occur together with the rescue of dendritic atrophy and reduction of spine number induced by chronic stress in the hippocampus and prefrontal cortex of animal models of depression. Nevertheless, the molecular mechanisms underlying these morphological alterations remain largely unknown. Here, we found that miR-9-5p levels were selectively reduced in the hippocampus of rats vulnerable to Chronic Mild Stress (CMS), while acute subanesthetic ketamine restored its levels to basal condition in just 24h; miR-9-5p expression inversely correlated with the anhedonic phenotype. A decrease of miR-9-5p was reproduced in an in vitro model of stress, based on primary hippocampal neurons incubated with the stress hormone corticosterone. In both CMS animals and primary neurons, decreased miR-9-5p levels were associated with dendritic simplification, while treatment with ketamine completely rescued the changes. In vitro modulation of miR-9-5p expression showed a direct role of miR-9-5p in regulating dendritic length and spine density in mature primary hippocampal neurons. Among the putative target genes tested, Rest and Sirt1 were validated as biological targets in primary neuronal cultures. Moreover, in line with miR-9-5p changes, REST protein expression levels were remarkably increased in both CMS vulnerable animals and corticosterone-treated neurons, while ketamine completely abolished this alteration. Finally, the shortening of dendritic length in corticosterone-treated neurons was shown to be partly rescued by miR-9-5p overexpression and dependent on REST protein expression. Overall, our data unveiled the functional role of miR-9-5p in the remodeling of dendritic arbor induced by stress/corticosterone in vulnerable animals and its rescue by acute antidepressant treatment with ketamine.
Keywords: CORT; Dendrite morphology; Ketamine; REST; Stress; miR-9-5p.
© 2021 Published by Elsevier Inc.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Involvement of miR-135a-5p Downregulation in Acute and Chronic Stress Response in the Prefrontal Cortex of Rats.Int J Mol Sci. 2023 Jan 13;24(2):1552. doi: 10.3390/ijms24021552. Int J Mol Sci. 2023. PMID: 36675068 Free PMC article.
-
Chronic mild stress induces anhedonic behavior and changes in glutamate release, BDNF trafficking and dendrite morphology only in stress vulnerable rats. The rapid restorative action of ketamine.Neurobiol Stress. 2019 Apr 2;10:100160. doi: 10.1016/j.ynstr.2019.100160. eCollection 2019 Feb. Neurobiol Stress. 2019. PMID: 31193464 Free PMC article.
-
Ketamine, but not fluoxetine, rapidly rescues corticosterone-induced impairments on glucocorticoid receptor and dendritic branching in the hippocampus of mice.Metab Brain Dis. 2021 Dec;36(8):2223-2233. doi: 10.1007/s11011-021-00743-2. Epub 2021 May 5. Metab Brain Dis. 2021. PMID: 33950381
-
Spine synapse remodeling in the pathophysiology and treatment of depression.Neurosci Lett. 2015 Aug 5;601:20-9. doi: 10.1016/j.neulet.2015.01.022. Epub 2015 Jan 9. Neurosci Lett. 2015. PMID: 25582786 Free PMC article. Review.
-
Fast-acting antidepressant activity of ketamine: highlights on brain serotonin, glutamate, and GABA neurotransmission in preclinical studies.Pharmacol Ther. 2019 Jul;199:58-90. doi: 10.1016/j.pharmthera.2019.02.017. Epub 2019 Mar 7. Pharmacol Ther. 2019. PMID: 30851296 Review.
Cited by
-
The Mechanisms Behind Rapid Antidepressant Effects of Ketamine: A Systematic Review With a Focus on Molecular Neuroplasticity.Front Psychiatry. 2022 Apr 25;13:860882. doi: 10.3389/fpsyt.2022.860882. eCollection 2022. Front Psychiatry. 2022. PMID: 35546951 Free PMC article.
-
Circulating Neuronal Exosome Cargo as Biomarkers of Neuroplasticity in Cushing's Syndrome.Mol Neurobiol. 2025 May 24. doi: 10.1007/s12035-025-05069-z. Online ahead of print. Mol Neurobiol. 2025. PMID: 40413304
-
MicroRNAs as biomarkers and molecular mediators of cognitive dysfunction in schizophrenia.J Neural Transm (Vienna). 2025 Aug 8. doi: 10.1007/s00702-025-02993-1. Online ahead of print. J Neural Transm (Vienna). 2025. PMID: 40779062 Review.
-
The Cellular Dysfunction of the Brain-Blood Barrier from Endothelial Cells to Astrocytes: The Pathway towards Neurotransmitter Impairment in Schizophrenia.Int J Mol Sci. 2024 Jan 19;25(2):1250. doi: 10.3390/ijms25021250. Int J Mol Sci. 2024. PMID: 38279249 Free PMC article. Review.
-
Human Adult Astrocyte Extracellular Vesicle Transcriptomics Study Identifies Specific RNAs Which Are Preferentially Secreted as EV Luminal Cargo.Genes (Basel). 2023 Mar 31;14(4):853. doi: 10.3390/genes14040853. Genes (Basel). 2023. PMID: 37107614 Free PMC article.
References
-
- Aceto G., Colussi C., Leone L., Fusco S., Rinaudo M., Scala F., Green T.A., Laezza F., D'Ascenzo M., Grassi C. Chronic mild stress alters synaptic plasticity in the nucleus accumbens through GSK3β-dependent modulation of Kv4.2 channels. Proc. Natl. Acad. Sci. Unit. States Am. 2020;117:8143–8153. doi: 10.1073/pnas.1917423117. - DOI - PMC - PubMed
-
- Andolina D., Di Segni M., Bisicchia E., D'Alessandro F., Cestari V., Ventura A., Concepcion C., Puglisi-Allegra S., Ventura R. Effects of lack of microRNA-34 on the neural circuitry underlying the stress response and anxiety. Neuropharmacology. 2016;107:305–316. doi: 10.1016/j.neuropharm.2016.03.044. - DOI - PMC - PubMed
-
- Bai Y.Y., Ruan C.S., Yang C.R., Li J, Kang Z-L, Zhou L, Liu D, Zeng Y-Q, Wang T-H, Tian C-F, Liao H, Bobrovskaya L, Zhou X-F. ProBDNF signaling regulates depression-like behaviors in rodents under chronic stress. Neuropsychopharmacology. 2016;41(12):2882–2892. doi: 10.1038/npp.2016.100. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

