COVID-19 Pandemic Spurs Evolution of an Academic Pathology Department and Laboratory
- PMID: 34458566
- PMCID: PMC8385575
- DOI: 10.1177/23742895211037029
COVID-19 Pandemic Spurs Evolution of an Academic Pathology Department and Laboratory
Abstract
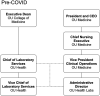
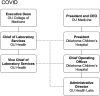
The COVID-19 pandemic has caused much suffering through disease and death, disruption of daily life, and economic havoc. Global health infrastructure has been challenged, in some cases failing. In the United States, the inability of laboratories to provide adequate testing for the causative pathogen, severe acute respiratory syndrome coronavirus 2, has been the subject of negative press and national debate. Even so, these challenges have prompted pathology practices and clinical labs to change their organizations and operations for the better. The natural positive evolution of the University of Oklahoma Department of Pathology and OU Health Laboratories has been greatly accelerated by the global pandemic. While developing a substantial COVID testing response, our department of pathology and laboratories have evolved a much nimbler organizational structure, established an important research partnership, built a translational research resource, created a significant reference lab capability, and completed many key hires against a national background of hiring freezes and pay cuts. Also, the high visibility of the clinical lab and pathologists during the outbreak has reinforced the value of lab medicine to patient care across our health system. In the midst of significant ongoing changes to the structure and financing of our underlying organizations, high trust among departmental, hospital, health system, and medical school leadership during the pandemic has promoted these positive changes, allowing us to emerge much stronger from this crisis.
Keywords: COVID-19; evolution; laboratory management; laboratory-developed test; severe acute respiratory syndrome coronavirus 2 polymerase chain reaction.
© The Author(s) 2021.
Conflict of interest statement
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Stitt JK. Third amended executive order 2020-07. Sos.ok.gov. 2020. Accessed December 8, 2020. https://www.sos.ok.gov/documents/executive/1917.pdf
-
- Sussman I, Prystowsky MB. Pathology service line: a model for accountable care organizations at an academic medical center. Hum Pathol. 2012;43:629–631. - PubMed
-
- Jensen KJ, Stallone R, Eller M, et al. Northwell health laboratories: the 10-year outcomes after deciding to keep the lab. Arch Pathol Lab Med. 2019;143:1517–1530. - PubMed
-
- Singhal S, Repasky C. The great acceleration in healthcare: six trends to heed. Mckinsey.com. 2020. Accessed May 8, 2021. https://www.mckinsey.com/industries/healthcare-systems-and-services/our-...
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous