Synthesis, single crystal (XRD), Hirshfeld surface analysis, computational study (DFT) and molecular docking studies of (E)-4-((2-hydroxy-3,5-diiodobenzylidene)amino)-N-(pyrimidine)-2-yl) benzenesulfonamide
- PMID: 34458601
- PMCID: PMC8379672
- DOI: 10.1016/j.heliyon.2021.e07724
Synthesis, single crystal (XRD), Hirshfeld surface analysis, computational study (DFT) and molecular docking studies of (E)-4-((2-hydroxy-3,5-diiodobenzylidene)amino)-N-(pyrimidine)-2-yl) benzenesulfonamide
Abstract
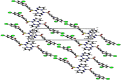
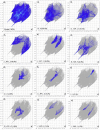
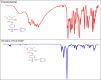
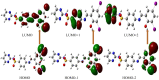
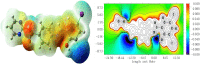
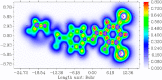
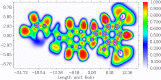
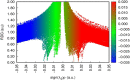
The Schiff base (E)-4-((2-hydroxy-3,5-diiodobenzylidene)amino)-N-(pyrimidine)-2-yl) benzene sulfonamide (DIDA) compound was synthesis with condensation of 3,5-diiodosalicylaldehyde and sulfadiazine. The compound characterized with FTIR, X-ray crystallography and electronic spectra. The titled compound associated with experimental and theoretical method, DFT used for the theoretical method. The IR was calculated from DFT mode with B3LYP/GENSEP basic set. The electronic spectra computed from TD-DFT method with CAM-B3LYP functional, with IEFPCM solvation model and DMSO used as the solvent. Wave function based properties like localized orbital locator, electron localization function and non-covalent interactions have been studied extensively. The ADMET properties of the compound DIDA indicated that the compound has excellent drug likeness properties and PASS studies showed that it has anti-infective properties, which is confirmed by a docking score of -7.4 kcal/mol.
Keywords: DFT; Drug-likeness; Molecular docking; Single crystal; Sulfadiazine; Synthesis.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
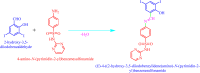
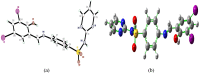


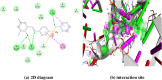
References
-
- Gadad A.K., Mahajanshetti C.S., Nimbalkar S., Raichurkar A. Synthesis and an- tibacterial activity of some 5-guanylhydrazone/thiocyanato-6-arylimidazo[2,1- b ]-1,3, 4-thiadiazole-2-sulfonamide derivatives. Eur. J. Med. Chem. 2000;35:853–857. - PubMed
-
- Maren T.H. Relatons between structure and biological activity of sulfonamides. Annu. Rev. Pharmacol. Toxicol. 1976;16:309–327. - PubMed
-
- Banuppriya G., Sribalan R., Padmini V. Synthesis and characterization of cur- cumin-sulfonamide hybrids: biological evaluation and molecular docking stud- ies. J. Mol. Struct. 2018;1155:90–100.
-
- Humphries P.S., Bersot R., Kincaid J., Mabery E., McCluskie K., Park T., Ren- ner T., Riegler E., Steinfeld T., Turtle E.D., Wei Z.L., Willis E. Carbazole-containing sulfonamides and sulfamides: discovery of cryptochrome modulators as antidi- abetic agents. Bioorg. Med. Chem. Lett. 2016;26:757–760. - PubMed
-
- Sun L., Wu Y., Liu Y., Chen X., Hu L. Novel carbazole sulfonamide derivatives of antitumor agent: synthesis, antiproliferative activity and aqueous solubility. Bioorg. Med. Chem. Lett. 2017;27:261–265. - PubMed
LinkOut - more resources
Full Text Sources

