Matters of class: coming of age of class III and IV lanthipeptides
- PMID: 34458752
- PMCID: PMC8341899
- DOI: 10.1039/d0cb00073f
Matters of class: coming of age of class III and IV lanthipeptides
Abstract
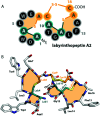
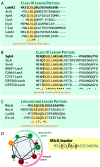
Lanthipeptides belong to the superfamily of ribosomally-synthesized and posttranslationally-modified peptides (RiPPs). Despite the fact that they represent one of the longest known RiPP subfamilies, their youngest members, classes III and IV, have only been described more recently. Since then, a plethora of studies furthered the understanding of their biosynthesis. While there are commonalities between classes III and IV due to the similar domain architectures of their processing enzymes, there are also striking differences that allow their discrimination. In this concise review article, we summarize what is known about the underlying biosynthetic principles of these lanthipeptides and discuss open questions for future research.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures









References
-
- Arnison P. G. Bibb M. J. Bierbaum G. Bowers A. A. Bugni T. S. Bulaj G. Camarero J. A. Campopiano D. J. Challis G. L. Clardy J. Cotter P. D. Craik D. J. Dawson M. Dittmann E. Donadio S. Dorrestein P. C. Entian K. D. Fischbach M. A. Garavelli J. S. Goransson U. Gruber C. W. Haft D. H. Hemscheidt T. K. Hertweck C. Hill C. Horswill A. R. Jaspars M. Kelly W. L. Klinman J. P. Kuipers O. P. Link A. J. Liu W. Marahiel M. A. Mitchell D. A. Moll G. N. Moore B. S. Muller R. Nair S. K. Nes I. F. Norris G. E. Olivera B. M. Onaka H. Patchett M. L. Piel J. Reaney M. J. Rebuffat S. Ross R. P. Sahl H. G. Schmidt E. W. Selsted M. E. Severinov K. Shen B. Sivonen K. Smith L. Stein T. Süssmuth R. D. Tagg J. R. Tang G. L. Truman A. W. Vederas J. C. Walsh C. T. Walton J. D. Wenzel S. C. Willey J. M. van der Donk W. A. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 2012;30(1):108. doi: 10.1039/C2NP20085F. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

