The chemical biology of coronavirus host-cell interactions
- PMID: 34458775
- PMCID: PMC8340996
- DOI: 10.1039/d0cb00197j
The chemical biology of coronavirus host-cell interactions
Abstract
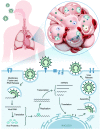
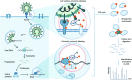
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the current coronavirus disease 2019 (COVID-19) pandemic that has led to a global economic disruption and collapse. With several ongoing efforts to develop vaccines and treatments for COVID-19, understanding the molecular interaction between the coronavirus, host cells, and the immune system is critical for effective therapeutic interventions. Greater insight into these mechanisms will require the contribution and combination of multiple scientific disciplines including the techniques and strategies that have been successfully deployed by chemical biology to tease apart complex biological pathways. We highlight in this review well-established strategies and methods to study coronavirus-host biophysical interactions and discuss the impact chemical biology will have on understanding these interactions at the molecular level.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
E. C. H., K. A. V., D. J. H., R. C. O., and O. O. F. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Figures





Similar articles
-
Proteomic Approaches to Study SARS-CoV-2 Biology and COVID-19 Pathology.J Proteome Res. 2021 Feb 5;20(2):1133-1152. doi: 10.1021/acs.jproteome.0c00764. Epub 2021 Jan 19. J Proteome Res. 2021. PMID: 33464917 Free PMC article.
-
Similarities and Dissimilarities of COVID-19 and Other Coronavirus Diseases.Annu Rev Microbiol. 2021 Oct 8;75:19-47. doi: 10.1146/annurev-micro-110520-023212. Epub 2021 Jan 25. Annu Rev Microbiol. 2021. PMID: 33492978
-
Molecular biology of the SARs-CoV-2 spike protein: A review of current knowledge.J Med Virol. 2021 Oct;93(10):5729-5741. doi: 10.1002/jmv.27132. Epub 2021 Jun 14. J Med Virol. 2021. PMID: 34125455 Free PMC article. Review.
-
Synergistic antiviral effect of hydroxychloroquine and azithromycin in combination against SARS-CoV-2: What molecular dynamics studies of virus-host interactions reveal.Int J Antimicrob Agents. 2020 Aug;56(2):106020. doi: 10.1016/j.ijantimicag.2020.106020. Epub 2020 May 13. Int J Antimicrob Agents. 2020. PMID: 32405156 Free PMC article.
-
Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19).J Pathol. 2020 Jul;251(3):228-248. doi: 10.1002/path.5471. Epub 2020 Jun 10. J Pathol. 2020. PMID: 32418199 Free PMC article. Review.
Cited by
-
Molecular Level Dissection of Critical Spike Mutations in SARS-CoV-2 Variants of Concern (VOCs): A Simplified Review.ChemistrySelect. 2021 Aug 20;6(31):7981-7998. doi: 10.1002/slct.202102074. Epub 2021 Aug 17. ChemistrySelect. 2021. PMID: 34541298 Free PMC article. Review.
-
COVID-19: Vaccines and therapeutics.Bioorg Med Chem Lett. 2022 Nov 1;75:128987. doi: 10.1016/j.bmcl.2022.128987. Epub 2022 Sep 14. Bioorg Med Chem Lett. 2022. PMID: 36113669 Free PMC article. Review.
-
Coinfection and Interference Phenomena Are the Results of Multiple Thermodynamic Competitive Interactions.Microorganisms. 2021 Sep 29;9(10):2060. doi: 10.3390/microorganisms9102060. Microorganisms. 2021. PMID: 34683381 Free PMC article.
References
-
- Corum J., Wu K. J. and Zimmer C., Coronavirus Drug and Treatment Tracker, nytimes.com/interactive/2020/science/coronavirus-drugs-treatments.html
-
- Yurkovetskiy L. Wang X. Pascal K. E. Tomkins-Tinch C. Nyalile T. P. Wang Y. Baum A. Diehl W. E. Dauphin A. Carbone C. Veinotte K. Egri S. B. Schaffner S. F. Lemieux J. E. Munro J. B. Rafique A. Barve A. Sabeti P. C. Kyratsous C. A. Dudkina N. V. Shen K. Luban J. Cell. 2020;183:739–751. doi: 10.1016/j.cell.2020.09.032. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

