Identifying cysteine residues susceptible to oxidation by photoactivatable atomic oxygen precursors using a proteome-wide analysis
- PMID: 34458801
- PMCID: PMC8341131
- DOI: 10.1039/d0cb00200c
Identifying cysteine residues susceptible to oxidation by photoactivatable atomic oxygen precursors using a proteome-wide analysis
Abstract
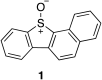
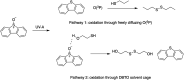
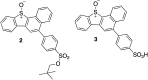
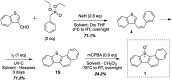
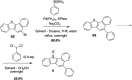
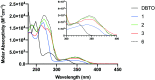
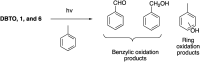
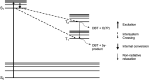
The reactivity profile of atomic oxygen [O(3P)] in the condensed phase has shown a preference for the thiol group of cysteines. In this work, water-soluble O(3P)-precursors were synthesized by adding aromatic burdens and water-soluble sulphonic acid groups to the core structure of dibenzothiophene-S-oxide (DBTO) to study O(3P) reactivity in cell lysates and live cells. The photodeoxygenation of these compounds was investigated using common intermediates, which revealed that an increase in aromatic burdens to the DBTO core structure decreases the total oxidation yield due to competitive photodeoxygenation mechanisms. These derivatives were then tested in cell lysates and live cells to profile changes in cysteine reactivity using the isoTOP-ABPP chemoproteomics platform. The results from this analysis indicated that O(3P) significantly affects cysteine reactivity in the cell. Additionally, O(3P) was found to oxidize cysteines within peptide sequences with leucine and serine conserved at the sites surrounding the oxidized cysteine. O(3P) was also found to least likely oxidize cysteines among membrane proteins.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Zadok E. Amar D. Mazur Y. J. Am. Chem. Soc. 1980;102:6369–6370. doi: 10.1021/ja00540a046. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

