Optical chemosensors for the detection of proximally phosphorylated peptides and proteins
- PMID: 34458812
- PMCID: PMC8341930
- DOI: 10.1039/d1cb00055a
Optical chemosensors for the detection of proximally phosphorylated peptides and proteins
Abstract
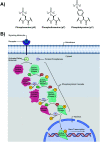
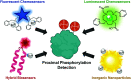
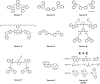
Proximal multi-site phosphorylation is a critical post-translational modification in protein biology. The additive effects of multiple phosphosite clusters in close spatial proximity triggers integrative and cooperative effects on protein conformation and activity. Proximal phosphorylation has been shown to modulate signal transduction pathways and gene expression, and as a result, is implicated in a broad range of disease states through altered protein function and/or localization including enzyme overactivation or protein aggregation. The role of proximal multi-phosphorylation events is becoming increasingly recognized as mechanistically important, although breakthroughs are limited due to a lack of detection technologies. To date, there is a limited selection of facile and robust sensing tools for proximal phosphorylation. Nonetheless, there have been considerable efforts in developing optical chemosensors for the detection of proximal phosphorylation motifs on peptides and proteins in recent years. This review provides a comprehensive overview of optical chemosensors for proximal phosphorylation, with the majority of work being reported in the past two decades. Optical sensors, in the form of fluorescent and luminescent chemosensors, hybrid biosensors, and inorganic nanoparticles, are described. Emphasis is placed on the rationale behind sensor scaffolds, relevant protein motifs, and applications in protein biology.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
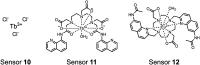

Similar articles
-
Structure-activity relationship study of ProxyPhos chemosensors for the detection of proximal phosphorylation and other phosphate species.Analyst. 2017 Oct 9;142(20):3922-3933. doi: 10.1039/c7an00722a. Analyst. 2017. PMID: 28930308
-
Characterization and application studies of ProxyPhos, a chemosensor for the detection of proximally phosphorylated peptides and proteins in aqueous solutions.Analyst. 2017 Jun 26;142(13):2451-2459. doi: 10.1039/c6an02537d. Analyst. 2017. PMID: 28574079
-
Molecular Probes, Chemosensors, and Nanosensors for Optical Detection of Biorelevant Molecules and Ions in Aqueous Media and Biofluids.Chem Rev. 2022 Feb 9;122(3):3459-3636. doi: 10.1021/acs.chemrev.1c00746. Epub 2022 Jan 7. Chem Rev. 2022. PMID: 34995461 Free PMC article. Review.
-
Self-immolative colorimetric, fluorescent and chemiluminescent chemosensors.Chem Soc Rev. 2018 Sep 17;47(18):6900-6916. doi: 10.1039/c7cs00841d. Chem Soc Rev. 2018. PMID: 30175338 Review.
-
Recent advancements in chemosensors for the detection of food spoilage.Food Chem. 2024 Mar 15;436:137733. doi: 10.1016/j.foodchem.2023.137733. Epub 2023 Oct 18. Food Chem. 2024. PMID: 37862988 Review.
Cited by
-
Label-Free Physical Techniques and Methodologies for Proteins Detection in Microfluidic Biosensor Structures.Biomedicines. 2022 Jan 18;10(2):207. doi: 10.3390/biomedicines10020207. Biomedicines. 2022. PMID: 35203416 Free PMC article. Review.
-
Magnetic relaxation switching assay based on three-dimensional assembly of Fe3O4@ZIF-8 for detection of cadmium ions.RSC Adv. 2022 Sep 2;12(38):25041-25047. doi: 10.1039/d2ra03926e. eCollection 2022 Aug 30. RSC Adv. 2022. PMID: 36199884 Free PMC article.
-
The Influence of Casein Coatings on the Corrosion Behavior of Mg-Based Alloys.Materials (Basel). 2022 Feb 14;15(4):1399. doi: 10.3390/ma15041399. Materials (Basel). 2022. PMID: 35207938 Free PMC article.
-
Zinc ion detection using a benzothiazole-based highly selective fluorescence "turn-on" chemosensor and its real-time application.RSC Adv. 2022 Sep 29;12(43):27839-27845. doi: 10.1039/d2ra04874d. eCollection 2022 Sep 28. RSC Adv. 2022. PMID: 36320258 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

