Opioid withdrawal produces sex-specific effects on fentanyl-vs.-food choice and mesolimbic transcription
- PMID: 34458885
- PMCID: PMC8389189
- DOI: 10.1016/j.bpsgos.2021.04.009
Opioid withdrawal produces sex-specific effects on fentanyl-vs.-food choice and mesolimbic transcription
Abstract
Background: Opioid withdrawal is a key driver of opioid addiction and an obstacle to recovery. However, withdrawal effects on opioid reinforcement and mesolimbic neuroadaptation are understudied and the role of sex is largely unknown.
Methods: Male (n=13) and female (n=12) rats responded under a fentanyl-vs.-food "choice" procedure during daily 2h sessions. In addition to the daily choice sessions, rats were provided extended access to fentanyl during 12h self-administration sessions. After two weeks of this self-administration regimen, the nucleus accumbens (NAc) and ventral tegmental area (VTA) of a subset of rats were subjected to RNA sequencing. In the remaining rats, a third week of this self-administration regimen was conducted, during which methadone effects on fentanyl-vs.-food choice were determined.
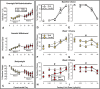
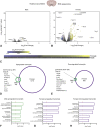
Results: Prior to opioid dependence, male and female rats similarly allocated responding between fentanyl and food. Abstinence from extended fentanyl access elicited similar increases in somatic withdrawal signs in both sexes. Despite similar withdrawal signs and extended access fentanyl intake, opioid withdrawal was accompanied by a maladaptive increase in fentanyl choice in males, but not females. Behavioral sex differences corresponded with a greater number of differentially expressed genes in the NAc and VTA of opioid-withdrawn females relative to males. Methadone blocked withdrawal-associated increases in fentanyl choice in males, but failed to further decrease fentanyl choice in females.
Conclusions: These results provide foundational evidence of sex-specific neuroadaptations to opioid withdrawal, which may be relevant to the female-specific resilience to withdrawal-associated increases in opioid choice and aid in the identification of novel therapeutic targets.
Keywords: Opioid withdrawal; RNA sequencing; decision making; nucleus accumbens; self-administration; sex differences.
Conflict of interest statement
5. Financial Disclosures The authors report no biomedical financial interests or potential conflicts of interest.
Figures



Similar articles
-
Conjugate vaccine produces long-lasting attenuation of fentanyl vs. food choice and blocks expression of opioid withdrawal-induced increases in fentanyl choice in rats.Neuropsychopharmacology. 2019 Sep;44(10):1681-1689. doi: 10.1038/s41386-019-0385-9. Epub 2019 May 2. Neuropsychopharmacology. 2019. PMID: 31043682 Free PMC article.
-
Sex Differences in the Development of an Opioid Addiction-Like Phenotype: A Focus on the Telescoping Effect.Biol Psychiatry Glob Open Sci. 2024 Aug 13;4(6):100373. doi: 10.1016/j.bpsgos.2024.100373. eCollection 2024 Nov. Biol Psychiatry Glob Open Sci. 2024. PMID: 39309210 Free PMC article.
-
Chronic inflammatory pain promotes place preference for fentanyl in male rats but does not change fentanyl self-administration in male and female rats.Neuropharmacology. 2023 Jun 15;231:109512. doi: 10.1016/j.neuropharm.2023.109512. Epub 2023 Mar 21. Neuropharmacology. 2023. PMID: 36948356 Free PMC article.
-
Modulation of drug choice by extended drug access and withdrawal in rhesus monkeys: Implications for negative reinforcement as a driver of addiction and target for medications development.Pharmacol Biochem Behav. 2018 Jan;164:32-39. doi: 10.1016/j.pbb.2017.04.006. Epub 2017 Apr 22. Pharmacol Biochem Behav. 2018. PMID: 28442370 Free PMC article. Review.
-
Focus on fentanyl in females: Sex and gender differences in the physiological and behavioral effects of fentanyl.Front Neuroendocrinol. 2023 Oct;71:101096. doi: 10.1016/j.yfrne.2023.101096. Epub 2023 Aug 18. Front Neuroendocrinol. 2023. PMID: 37597668 Review.
Cited by
-
Mu Opioid Receptor-Positive Neurons in the Dorsal Raphe Nucleus Are Impaired by Morphine Abstinence.Biol Psychiatry. 2023 Dec 1;94(11):852-862. doi: 10.1016/j.biopsych.2023.06.024. Epub 2023 Jun 29. Biol Psychiatry. 2023. PMID: 37393045 Free PMC article.
-
Transcriptional signatures of fentanyl use in the mouse ventral tegmental area.Addict Biol. 2024 May;29(5):e13403. doi: 10.1111/adb.13403. Addict Biol. 2024. PMID: 38735880 Free PMC article.
-
Effect of TRV130 and methadone on fentanyl-vs.-food choice and somatic withdrawal signs in opioid-dependent and post-opioid-dependent rats.Neuropsychopharmacology. 2022 Nov;47(12):2132-2139. doi: 10.1038/s41386-022-01393-3. Epub 2022 Jul 29. Neuropsychopharmacology. 2022. PMID: 35906489 Free PMC article.
-
Lack of effect of the nociceptin opioid peptide agonist Ro 64-6198 on pain-depressed behavior and heroin choice in rats.Drug Alcohol Depend. 2022 Feb 1;231:109255. doi: 10.1016/j.drugalcdep.2021.109255. Epub 2021 Dec 30. Drug Alcohol Depend. 2022. PMID: 34998256 Free PMC article.
-
Evidence for Endogenous Opioid Dependence Related to Latent Sensitization in a Rat Model of Chronic Inflammatory Pain.Int J Mol Sci. 2023 Feb 1;24(3):2812. doi: 10.3390/ijms24032812. Int J Mol Sci. 2023. PMID: 36769126 Free PMC article.
References
-
- BlueCross BlueShield America’s Opioid Epidemic and Its Effect on the Nation’s Commercially Insured Population. The Health of America Report. 2017 https://www.bcbs.com/sites/default/files/file-attachments/health-of-amer... Available at: Accessed March 23, 2021.
-
- US Food and Drug Administration, Center for Drug Evaluation and Research The Voice of the Patient. Opioid Use Disorder. 2018 https://www.fda.gov/media/124391/download Available at: Accessed March 20, 2021.
-
- Thompson T., Schuster C.R. Morphine self-administration, food-reinforced, and avoidance behaviors in rhesus monkeys. Psychopharmacologia. 1964;5:87–94. - PubMed
-
- Yanagita T. Drug dependence studies in laboratory animals. NIDA Res Monogr. 1978;19:179–190. - PubMed
-
- Carrera M.R., Schulteis G., Koob G.F. Heroin self-administration in dependent Wistar rats: Increased sensitivity to naloxone. Psychopharmacology (Berl) 1999;144:111–120. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

