Potential test-negative design study bias in outbreak settings: application to Ebola vaccination in Democratic Republic of Congo
- PMID: 34458913
- PMCID: PMC8855996
- DOI: 10.1093/ije/dyab172
Potential test-negative design study bias in outbreak settings: application to Ebola vaccination in Democratic Republic of Congo
Abstract
Background: Infectious disease outbreaks present unique challenges to study designs for vaccine evaluation. Test-negative design (TND) studies have previously been used to estimate vaccine effectiveness and have been proposed for Ebola virus disease (EVD) vaccines. However, there are key differences in how cases and controls are recruited during outbreaks and pandemics of novel pathogens, whcih have implications for the reliability of effectiveness estimates using this design.
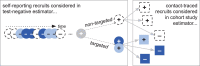
Methods: We use a modelling approach to quantify TND bias for a prophylactic vaccine under varying study and epidemiological scenarios. Our model accounts for heterogeneity in vaccine distribution and for two potential routes to testing and recruitment into the study: self-reporting and contact-tracing. We derive conventional and hybrid TND estimators for this model and suggest ways to translate public health response data into the parameters of the model.
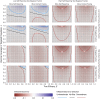
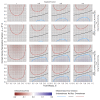
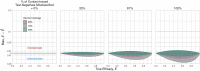
Results: Using a conventional TND study, our model finds biases in vaccine effectiveness estimates. Bias arises due to differential recruitment from self-reporting and contact-tracing, and due to clustering of vaccination. We estimate the degree of bias when recruitment route is not available, and propose a study design to eliminate the bias if recruitment route is recorded.
Conclusions: Hybrid TND studies can resolve the design bias with conventional TND studies applied to outbreak and pandemic response testing data, if those efforts collect individuals' routes to testing. Without route to testing, other epidemiological data will be required to estimate the magnitude of potential bias in a conventional TND study. Since these studies may need to be conducted retrospectively, public health responses should obtain these data, and generic protocols for outbreak and pandemic response studies should emphasize the need to record routes to testing.
Keywords: DRC; Ebola; Test-negative design; mathematical modelling; outbreak response.
© The Author(s) 2021. Published by Oxford University Press on behalf of the International Epidemiological Association.
Figures



References
-
- Baden LR, Rubin EJ, Morrissey S, Farrar JJ, Drazen JM.. We can do better - improving outcomes in the midst of an emergency. N Engl J Med 2017;377:1482–84. - PubMed
-
- WHO. Vaccination in Acute Humanitarian Emergencies: A Framework for Decision Making. Geneva: World Health Organization, 2017.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

