COVID-19: potential therapeutics for pediatric patients
- PMID: 34458951
- PMCID: PMC8403523
- DOI: 10.1007/s43440-021-00316-1
COVID-19: potential therapeutics for pediatric patients
Abstract
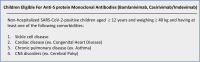
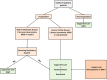
The global spread of COVID-19 has imparted significant economic, medical, and social burdens. Like adults, children are affected by this pandemic. However, milder clinical symptoms are often experienced by them. Only a minimal proportion of the affected patients may develop severe and complicated COVID-19. Supportive treatment is recommended in all patients. Antiviral and immunomodulatory medications are spared for hospitalized children with respiratory distress or severe to critical disease. Up till now, remdesivir is the only USFDA-approved anti-COVID-19 medication indicated in the majority of symptomatic patients with moderate to severe disease. Dexamethasone is solely recommended in patients with respiratory distress maintained on oxygen or ventilatory support. The use of these medications in pediatric patients is founded on evidence deriving from adult studies. No randomized controlled trials (RCTs) involving pediatric COVID-19 patients have assessed these medications' efficacy and safety, among others. Similarly, three novel monoclonal anti-SARS-CoV-2 spike protein antibodies, bamlanivimab, casirivimab and imdevimab, have been recently authorized by the USFDA. Nonetheless, their efficacy has not been demonstrated by multiple RCTs. In this review, we aim to dissect the various potential therapeutics used in children with COVID-19. We aspire to provide a comprehensive review of the available evidence and display the mechanisms of action and the pharmacokinetic properties of the studied therapeutics. Our review offers an efficient and practical guide for treating children with COVID-19.
Keywords: COVID-19; Pediatric patients; SARS-CoV-2; Therapeutics.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Younis NK, Zareef RO, Mohammad Ali NM, Maktabi R. The era of the coronavirus disease 2019 pandemic: a review on dynamics, clinical symptoms and complications, diagnosis, and treatment. Genetic Testing Molecular Biomarkers. 2021;25:85–101. - PubMed
-
- Younis NK, Rahm M, Bitar F, Arabi M. COVID-19 in the MENA region: facts and findings. J Infect Dev Ctries. 2021;15:342–349. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
