Physiologically Based Pharmacokinetic Modelling of Inhaled Nemiralisib: Mechanistic Components for Pulmonary Absorption, Systemic Distribution, and Oral Absorption
- PMID: 34458976
- PMCID: PMC8813803
- DOI: 10.1007/s40262-021-01066-2
Physiologically Based Pharmacokinetic Modelling of Inhaled Nemiralisib: Mechanistic Components for Pulmonary Absorption, Systemic Distribution, and Oral Absorption
Abstract
Background and objectives: Physiologically based pharmacokinetic (PBPK) modelling has evolved to accommodate different routes of drug administration and enables prediction of drug concentrations in tissues as well as plasma. The inhalation route of administration has proven successful in treating respiratory diseases but can also be used for rapid systemic delivery, holding great promise for treatment of diseases requiring systemic exposure. The objective of this work was to develop a PBPK model that predicts plasma and tissue concentrations following inhalation administration of the PI3Kδ inhibitor nemiralisib.
Methods: A PBPK model was built in GastroPlus® that includes a complete mechanistic description of pulmonary absorption, systemic distribution and oral absorption following inhalation administration of nemiralisib. The availability of clinical data obtained after intravenous, oral and inhalation administration enabled validation of the model with observed data and accurate assessment of pulmonary drug absorption. The PBPK model described in this study incorporates novel use of key parameters such as lung systemic absorption rate constants derived from human physiological lung blood flows, and implementation of the specific permeability-surface area product per millilitre of tissue cell volume (SpecPStc) to predict tissue distribution.
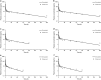
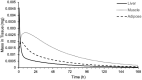
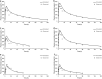
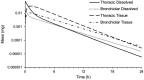
Results: The inhaled PBPK model was verified using plasma and bronchoalveolar lavage fluid concentration data obtained in human subjects. Prediction of tissue concentrations using the permeability-limited systemic disposition tissue model was further validated using tissue concentration data obtained in the rat following intravenous infusion administration to steady state.
Conclusions: Fully mechanistic inhaled PBPK models such as the model described herein could be applied for cross molecule assessments with respect to lung retention and systemic exposure, both in terms of pharmacology and toxicology, and may facilitate clinical indication selection.
© 2021. The Author(s).
Conflict of interest statement
Chris D. Edwards, Augustin Amour, Ed Taylor, Olivia Robb, Brett O’Brien, Aarti Patel and Andrew W. Harrell are employees of GlaxoSmithKline R&D and may hold stock or stock options. Neil A. Miller, Rebecca H. Graves & Edith M. Hessel were employees of GlaxoSmithKline R&D at the time this work was completed and may hold stock. Neil A. Miller and Rebecca H. Graves are now employees of Simulations Plus (UK) and may hold stock options and Edith M. Hessel is Chief Scientific Officer at Eligo Bioscience (Paris, France).
Figures


References
-
- Gupta V, Khan A, Higham A, Lemon J, Sriskantharajah S, Amour A, et al. The effect of phosphatidylinositol-3 kinase inhibition on matrix metalloproteinase-9 and reactive oxygen species release from chronic obstructive pulmonary disease neutrophils. Int Immunopharmacol. 2016;5:155–162. doi: 10.1016/j.intimp.2016.03.027. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

