Effect of Empagliflozin on Worsening Heart Failure Events in Patients With Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial
- PMID: 34459213
- PMCID: PMC8522627
- DOI: 10.1161/CIRCULATIONAHA.121.056824
Effect of Empagliflozin on Worsening Heart Failure Events in Patients With Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial
Abstract
Background: Empagliflozin reduces the risk of cardiovascular death or hospitalization for heart failure in patients with heart failure with preserved ejection fraction, but additional data are needed about its effect on inpatient and outpatient heart failure events.
Methods: We randomly assigned 5988 patients with class II through IV heart failure with an ejection fraction of >40% to double-blind treatment with placebo or empagliflozin (10 mg once daily), in addition to usual therapy, for a median of 26 months. We prospectively collected information on inpatient and outpatient events reflecting worsening heart failure and prespecified their analysis in individual and composite end points.
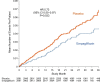
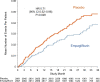
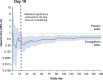
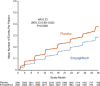
Results: Empagliflozin reduced the combined risk of cardiovascular death, hospitalization for heart failure, or an emergency or urgent heart failure visit requiring intravenous treatment (432 versus 546 patients [empagliflozin versus placebo, respectively]; hazard ratio, 0.77 [95% CI, 0.67-0.87]; P<0.0001). This benefit reached statistical significance at 18 days after randomization. Empagliflozin reduced the total number of heart failure hospitalizations that required intensive care (hazard ratio, 0.71 [95% CI, 0.52-0.96]; P=0.028) and the total number of all hospitalizations that required a vasopressor or positive inotropic drug (hazard ratio, 0.73 [95% CI, 0.55-0.97]; P=0.033). Compared with patients in the placebo group, fewer patients in the empagliflozin group reported outpatient intensification of diuretics (482 versus 610; hazard ratio, 0.76 [95% CI, 0.67-0.86]; P<0.0001), and patients assigned to empagliflozin were 20% to 50% more likely to have a better New York Heart Association functional class, with significant effects at 12 weeks that were maintained for at least 2 years. The benefit on total heart failure hospitalizations was similar in patients with an ejection fraction of >40% to <50% and 50% to <60%, but was attenuated at higher ejection fractions.
Conclusions: In patients with heart failure with preserved ejection fraction, empagliflozin produced a meaningful, early, and sustained reduction in the risk and severity of a broad range of inpatient and outpatient worsening heart failure events. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT03057977.
Keywords: heart failure; sodium-glucose transporter 2 inhibitors.
Figures
Comment in
-
Letter by Gronda et al Regarding Article, "Effect of Empagliflozin on Worsening Heart Failure Events in Patients With Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial".Circulation. 2022 Apr 19;145(16):e839-e840. doi: 10.1161/CIRCULATIONAHA.121.057530. Epub 2022 Apr 18. Circulation. 2022. PMID: 35436134 No abstract available.
References
-
- Tyl B, Lopez Sendon J, Borer JS, Lopez De Sa E, Lerebours G, Varin C, De Montigny A, Pannaux M, Komajda M. Comparison of outcome adjudication by investigators and by a central end point committee in heart failure trials: experience of the SHIFT heart failure study. Circ Heart Fail. 2020; 13:e006720. doi: 10.1161/CIRCHEARTFAILURE.119.006720 - PubMed
-
- Carson P, Fiuzat M, O’Connor C, Anand I, Plehn J, Lindenfeld JA, Silver M, White M, Miller A, Davis G, et al. . Determination of hospitalization type by investigator case report form or adjudication committee in a large heart failure clinical trial (β-Blocker Evaluation of Survival Trial [BEST]). Am Heart J. 2010; 160:649–654. doi: 10.1016/j.ahj.2010.07.004 - PubMed
-
- Cleland JG. How to assess new treatments for the management of heart failure: composite scoring systems to assess the patients’ clinical journey. Eur J Heart Fail. 2002; 4:243–247. doi: 10.1016/s1388-9842(02)00039-9 - PubMed
-
- Cleland JG, Charlesworth A, Lubsen J, Swedberg K, Remme WJ, Erhardt L, Di Lenarda A, Komajda M, Metra M, Torp-Pedersen C, et al. ; COMET Investigators. A comparison of the effects of carvedilol and metoprolol on well-being, morbidity, and mortality (the “patient journey”) in patients with heart failure: a report from the Carvedilol or Metoprolol European Trial (COMET). J Am Coll Cardiol. 2006; 47:1603–1611. doi: 10.1016/j.jacc.2005.11.069 - PubMed
-
- Mallick A, Gandhi PU, Gaggin HK, Ibrahim N, Januzzi JL. The importance of worsening heart failure in ambulatory patients: definition, characteristics, and effects of amino-terminal pro-B-type natriuretic peptide guided therapy. JACC Heart Fail. 2016; 4:749–755. doi: 10.1016/j.jchf.2016.03.012 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

