Accelerated developmental adipogenesis programs adipose tissue dysfunction and cardiometabolic risk in offspring born to dams with metabolic dysfunction
- PMID: 34459218
- PMCID: PMC8791794
- DOI: 10.1152/ajpendo.00229.2021
Accelerated developmental adipogenesis programs adipose tissue dysfunction and cardiometabolic risk in offspring born to dams with metabolic dysfunction
Abstract
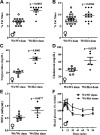
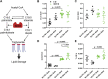
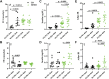
This study determined if a perturbation in in utero adipogenesis leading to later life adipose tissue (AT) dysfunction underlies programming of cardiometabolic risk in offspring born to dams with metabolic dysfunction. Female mice heterozygous for the leptin receptor deficiency (Hetdb) had 2.4-fold higher prepregnancy fat mass and in late gestation had higher plasma insulin and triglycerides compared with wild-type (Wt) females (P < 0.05). To isolate the role of the intrauterine milieu, wild-type (Wt) offspring from each pregnancy were studied. Differentiation potential in isolated progenitors and cell size distribution analysis revealed accelerated adipogenesis in Wt pups born to Hetdb dams, accompanied by a higher accumulation of neonatal fat mass. In adulthood, whole body fat mass by NMR was higher in male (69%) and female (20%) Wt offspring born to Hetdb versus Wt pregnancies, along with adipocyte hypertrophy and hyperlipidemia (all P < 0.05). Lipidomic analyses by gas chromatography revealed an increased lipogenic index (16:0/18:2n6) after high-fat/fructose diet (HFFD). Postprandial insulin, ADIPO-IR, and ex vivo AT lipolytic responses to isoproterenol were all higher in Wt offspring born to Hetdb dams (P < 0.05). Intrauterine metabolic stimuli may direct a greater proportion of progenitors toward terminal differentiation, thereby predisposing to hypertrophy-induced adipocyte dysfunction.NEW & NOTEWORTHY This study reveals that accelerated adipogenesis during the perinatal window of adipose tissue development predisposes to later life hypertrophic adipocyte dysfunction, thereby compromising the buffering function of the subcutaneous depot.
Keywords: adipogenesis; developmental programming; lipogenesis; programming.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


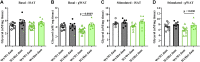

References
-
- Sacks DA, Hadden DR, Maresh M, Deerochanawong C, Dyer AR, Metzger BE, Lowe LP, Coustan DR, Hod M, Oats JJ, Persson B, Trimble ER; HAPO Study Cooperative Research Group. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care 35: 526–528, 2012. doi:10.2337/dc11-1641. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

