Robust humoral and cellular immune responses and low risk for reinfection at least 8 months following asymptomatic to mild COVID-19
- PMID: 34459525
- PMCID: PMC8661920
- DOI: 10.1111/joim.13387
Robust humoral and cellular immune responses and low risk for reinfection at least 8 months following asymptomatic to mild COVID-19
Abstract
Background: Emerging data support detectable immune responses for months after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and vaccination, but it is not yet established to what degree and for how long protection against reinfection lasts.
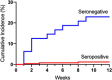
Methods: We investigated SARS-CoV-2-specific humoral and cellular immune responses more than 8 months post-asymptomatic, mild and severe infection in a cohort of 1884 healthcare workers (HCW) and 51 hospitalized COVID-19 patients. Possible protection against SARS-CoV-2 reinfection was analyzed by a weekly 3-month polymerase chain reaction (PCR) screening of 252 HCW that had seroconverted 7 months prior to start of screening and 48 HCW that had remained seronegative at multiple time points.
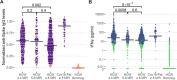
Results: All COVID-19 patients and 96% (355/370) of HCW who were anti-spike IgG positive at inclusion remained anti-spike IgG positive at the 8-month follow-up. Circulating SARS-CoV-2-specific memory T cell responses were detected in 88% (45/51) of COVID-19 patients and in 63% (233/370) of seropositive HCW. The cumulative incidence of PCR-confirmed SARS-CoV-2 infection was 1% (3/252) among anti-spike IgG positive HCW (0.13 cases per 100 weeks at risk) compared to 23% (11/48) among anti-spike IgG negative HCW (2.78 cases per 100 weeks at risk), resulting in a protective effect of 95.2% (95% CI 81.9%-99.1%).
Conclusions: The vast majority of anti-spike IgG positive individuals remain anti-spike IgG positive for at least 8 months regardless of initial COVID-19 disease severity. The presence of anti-spike IgG antibodies is associated with a substantially reduced risk of reinfection up to 9 months following asymptomatic to mild COVID-19.
Keywords: COVID-19; SARS-CoV-2; humoral response; long-term immunity; reinfection.
© 2021 The Authors. Journal of Internal Medicine published by John Wiley & Sons Ltd on behalf of Association for Publication of The Journal of Internal Medicine.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection.EBioMedicine. 2021 Sep;71:103561. doi: 10.1016/j.ebiom.2021.103561. Epub 2021 Aug 27. EBioMedicine. 2021. PMID: 34455390 Free PMC article.
-
Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers.N Engl J Med. 2021 Feb 11;384(6):533-540. doi: 10.1056/NEJMoa2034545. Epub 2020 Dec 23. N Engl J Med. 2021. PMID: 33369366 Free PMC article.
-
Risk Factors for Being Seronegative following SARS-CoV-2 Infection in a Large Cohort of Health Care Workers in Denmark.Microbiol Spectr. 2021 Oct 31;9(2):e0090421. doi: 10.1128/Spectrum.00904-21. Epub 2021 Oct 20. Microbiol Spectr. 2021. PMID: 34668738 Free PMC article.
-
Recovery scenario and immunity in COVID-19 disease: A new strategy to predict the potential of reinfection.J Adv Res. 2021 Jul;31:49-60. doi: 10.1016/j.jare.2020.12.013. Epub 2021 Jan 5. J Adv Res. 2021. PMID: 33520309 Free PMC article. Review.
-
Humoral Responses and Serological Assays in SARS-CoV-2 Infections.Front Immunol. 2020 Dec 18;11:610688. doi: 10.3389/fimmu.2020.610688. eCollection 2020. Front Immunol. 2020. PMID: 33391281 Free PMC article. Review.
Cited by
-
Transient production of receptor-binding domain of SARS-CoV-2 in Nicotiana benthamiana plants induces specific antibodies in immunized mice.Mol Biol Rep. 2022 Jul;49(7):6113-6123. doi: 10.1007/s11033-022-07402-4. Epub 2022 May 8. Mol Biol Rep. 2022. PMID: 35526244 Free PMC article.
-
Immune Response 5-7 Months after Vaccination against SARS-CoV-2 in Elderly Nursing Home Residents in the Czech Republic: Comparison of Three Vaccines.Viruses. 2022 May 18;14(5):1086. doi: 10.3390/v14051086. Viruses. 2022. PMID: 35632827 Free PMC article.
-
Effects of COVID-19 vaccination and previous infection on Omicron SARS-CoV-2 infection and relation with serology.Nat Commun. 2023 Aug 9;14(1):4793. doi: 10.1038/s41467-023-40195-z. Nat Commun. 2023. PMID: 37558656 Free PMC article.
-
Salivary Antibody Responses to Two COVID-19 Vaccines following Different Vaccination Regimens.Vaccines (Basel). 2023 Mar 28;11(4):744. doi: 10.3390/vaccines11040744. Vaccines (Basel). 2023. PMID: 37112657 Free PMC article.
-
Role of T cells in severe COVID-19 disease, protection, and long term immunity.Immunogenetics. 2023 Jun;75(3):295-307. doi: 10.1007/s00251-023-01294-9. Epub 2023 Feb 8. Immunogenetics. 2023. PMID: 36752852 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

