Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and mRNA-1273
- PMID: 34459863
- PMCID: PMC8406205
- DOI: 10.1001/jama.2021.15125
Comparison of SARS-CoV-2 Antibody Response Following Vaccination With BNT162b2 and mRNA-1273
Abstract
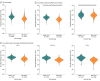
This study compares the immune responses to the BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) COVID-19 vaccines in health care workers in Belgium.
Conflict of interest statement
Figures
References
-
- Voysey M, Costa Clemens SA, Madhi SA, et al. ; Oxford COVID Vaccine Trial Group . Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881-891. doi: 10.1016/S0140-6736(21)00432-3 - DOI - PMC - PubMed
-
- Rubio-Acero R, Castelletti N, Fingerle V, et al. ; KoCo19 study team . In search of the SARS-CoV-2 protection correlate: head-to-head comparison of two quantitative S1 assays in pre-characterized oligo-/asymptomatic patients. Infect Dis Ther. 2021;10(3):1505-1518. doi: 10.1007/s40121-021-00475-x - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

