Emerging Neuroimaging Biomarkers Across Disease Stage in Parkinson Disease: A Review
- PMID: 34459865
- PMCID: PMC9017381
- DOI: 10.1001/jamaneurol.2021.1312
Emerging Neuroimaging Biomarkers Across Disease Stage in Parkinson Disease: A Review
Abstract
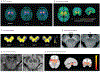
Importance: Imaging biomarkers in Parkinson disease (PD) are increasingly important for monitoring progression in clinical trials and also have the potential to improve clinical care and management. This Review addresses a critical need to make clear the temporal relevance for diagnostic and progression imaging biomarkers to be used by clinicians and researchers over the clinical course of PD. Magnetic resonance imaging (diffusion imaging, neuromelanin-sensitive imaging, iron-sensitive imaging, T1-weighted imaging), positron emission tomography/single-photon emission computed tomography dopaminergic, serotonergic, and cholinergic imaging as well as metabolic and cerebral blood flow network neuroimaging biomarkers in the preclinical, prodromal, early, and moderate to late stages are characterized.
Observations: If a clinical trial is being carried out in the preclinical and prodromal stages, potentially useful disease-state biomarkers include dopaminergic imaging of the striatum; metabolic imaging; free-water, neuromelanin-sensitive, and iron-sensitive imaging in the substantia nigra; and T1-weighted structural magnetic resonance imaging. Disease-state biomarkers that can distinguish atypical parkinsonisms are metabolic imaging, free-water imaging, and T1-weighted imaging; dopaminergic imaging and other molecular imaging track progression in prodromal patients, whereas other established progression biomarkers need to be evaluated in prodromal cohorts. Progression in early-stage PD can be monitored using dopaminergic imaging in the striatum, metabolic imaging, and free-water and neuromelanin-sensitive imaging in the posterior substantia nigra. Progression in patients with moderate to late-stage PD can be monitored using free-water imaging in the anterior substantia nigra, R2* of substantia nigra, and metabolic imaging. Cortical thickness and gyrification might also be useful markers or predictors of progression. Dopaminergic imaging and free-water imaging detect progression over 1 year, whereas other modalities detect progression over 18 months or longer. The reliability of progression biomarkers varies with disease stage, whereas disease-state biomarkers are relatively consistent in individuals with preclinical, prodromal, early, and moderate to late-stage PD.
Conclusions and relevance: Imaging biomarkers for various stages of PD are readily available to be used as outcome measures in clinical trials and are potentially useful in multimodal combination with routine clinical assessment. This Review provides a critically important template for considering disease stage when implementing diagnostic and progression biomarkers in both clinical trials and clinical care settings.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

