G-quadruplex DNA: a novel target for drug design
- PMID: 34459951
- PMCID: PMC11072987
- DOI: 10.1007/s00018-021-03921-8
G-quadruplex DNA: a novel target for drug design
Abstract
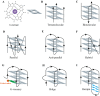
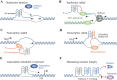
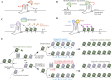
G-quadruplex (G4) DNA is a type of quadruple helix structure formed by a continuous guanine-rich DNA sequence. Emerging evidence in recent years authenticated that G4 DNA structures exist both in cell-free and cellular systems, and function in different diseases, especially in various cancers, aging, neurological diseases, and have been considered novel promising targets for drug design. In this review, we summarize the detection method and the structure of G4, highlighting some non-canonical G4 DNA structures, such as G4 with a bulge, a vacancy, or a hairpin. Subsequently, the functions of G4 DNA in physiological processes are discussed, especially their regulation of DNA replication, transcription of disease-related genes (c-MYC, BCL-2, KRAS, c-KIT et al.), telomere maintenance, and epigenetic regulation. Typical G4 ligands that target promoters and telomeres for drug design are also reviewed, including ellipticine derivatives, quinoxaline analogs, telomestatin analogs, berberine derivatives, and CX-5461, which is currently in advanced phase I/II clinical trials for patients with hematologic cancer and BRCA1/2-deficient tumors. Furthermore, since the long-term stable existence of G4 DNA structures could result in genomic instability, we summarized the G4 unfolding mechanisms emerged recently by multiple G4-specific DNA helicases, such as Pif1, RecQ family helicases, FANCJ, and DHX36. This review aims to present a general overview of the field of G-quadruplex DNA that has progressed in recent years and provides potential strategies for drug design and disease treatment.
Keywords: Aging; Cancer; DNA replication; Epigenetic; G-quadruplex; G4 ligand; Helicase; Telomere.
© 2021. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

