Reversal of SARS-CoV2-Induced Hypoxia by Nebulized Sodium Ibuprofenate in a Compassionate Use Program
- PMID: 34460083
- PMCID: PMC8404026
- DOI: 10.1007/s40121-021-00527-2
Reversal of SARS-CoV2-Induced Hypoxia by Nebulized Sodium Ibuprofenate in a Compassionate Use Program
Abstract
Introduction: Sodium ibuprofenate in hypertonic saline (NaIHS) administered directly to the lungs by nebulization and inhalation has antibacterial and anti-inflammatory effects, with the potential to deliver these benefits to hypoxic patients. We describe a compassionate use program that offered this therapy to hospitalized COVID-19 patients.
Methods: NaIHS (50 mg ibuprofen, tid) was provided in addition to standard of care (SOC) to hospitalized COVID-19 patients until oxygen saturation levels of > 94% were achieved on ambient air. Patients wore a containment hood to diminish aerosolization. Outcome data from participating patients treated at multiple hospitals in Argentina between April 4 and October 31, 2020, are summarized. Results were compared with a retrospective contemporaneous control (CC) group of hospitalized COVID-19 patients with SOC alone during the same time frame from a subset of participating hospitals from Córdoba and Buenos Aires.
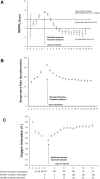
Results: The evolution of 383 patients treated with SOC + NaIHS [56 on mechanical ventilation (MV) at baseline] and 195 CC (21 on MV at baseline) are summarized. At baseline, NaIHS-treated patients had basal oxygen saturation of 90.7 ± 0.2% (74.3% were on supplemental oxygen at baseline) and a basal respiratory rate of 22.7 ± 0.3 breath/min. In the CC group, basal oxygen saturation was 92.6 ± 0.4% (52.1% were on oxygen supplementation at baseline) and respiratory rate was 19.3 ± 0.3 breath/min. Despite greater pulmonary compromise at baseline in the NaIHS-treated group, the length of treatment (LOT) was 9.1 ± 0.2 gs with an average length of stay (ALOS) of 11.5 ± 0.3 days, in comparison with an ALOS of 13.3 ± 0.9 days in the CC group. In patients on MV who received NaIHS, the ALOS was lower than in the CC group. In both NaIHS-treated groups, a rapid reversal of deterioration in oxygenation and NEWS2 scores was observed acutely after initiation of NaIHS therapy. No serious adverse events were considered related to ibuprofen therapy. Mortality was lower in both NaIHS groups compared with CC groups.
Conclusions: Treatment of COVID-19 pneumonitis with inhalational nebulized NaIHS was associated with rapid improvement in hypoxia and vital signs, with no serious adverse events attributed to therapy. Nebulized NaIHS s worthy of further study in randomized, placebo-controlled trials (ClinicalTrials.gov: NCT04382768).
Keywords: Acute respiratory distress syndrome; COVID-19; Coronavirus; Hypoxemia; SARS-CoV-2; Viral pneumonia.
© 2021. The Author(s).
Conflict of interest statement
All named authors confirm that they have no conflicts of interest to declare.
Figures

References
-
- WHO Coronavirus (COVID-19) Dashboard [Internet]. https://covid19.who.int. Accessed 2021 June 29.
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

