Effect of Immunosuppression on the Immunogenicity of mRNA Vaccines to SARS-CoV-2 : A Prospective Cohort Study
- PMID: 34461029
- PMCID: PMC8407518
- DOI: 10.7326/M21-1757
Effect of Immunosuppression on the Immunogenicity of mRNA Vaccines to SARS-CoV-2 : A Prospective Cohort Study
Abstract
Background: Patients with chronic inflammatory disease (CID) treated with immunosuppressive medications have increased risk for severe COVID-19. Although mRNA-based SARS-CoV-2 vaccination provides protection in immunocompetent persons, immunogenicity in immunosuppressed patients with CID is unclear.
Objective: To determine the immunogenicity of mRNA-based SARS-CoV-2 vaccines in patients with CID.
Design: Prospective observational cohort study.
Setting: Two U.S. CID referral centers.
Participants: Volunteer sample of adults with confirmed CID eligible for early COVID-19 vaccination, including hospital employees of any age and patients older than 65 years. Immunocompetent participants were recruited separately from hospital employees. All participants received 2 doses of mRNA vaccine against SARS-CoV-2 between 10 December 2020 and 20 March 2021. Participants were assessed within 2 weeks before vaccination and 20 days after final vaccination.
Measurements: Anti-SARS-CoV-2 spike (S) IgG+ binding in all participants, and neutralizing antibody titers and circulating S-specific plasmablasts in a subset to assess humoral response after vaccination.
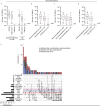
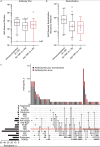
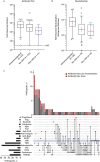
Results: Most of the 133 participants with CID (88.7%) and all 53 immunocompetent participants developed antibodies in response to mRNA-based SARS-CoV-2 vaccination, although some with CID developed numerically lower titers of anti-S IgG. Anti-S IgG antibody titers after vaccination were lower in participants with CID receiving glucocorticoids (n = 17) than in those not receiving them; the geometric mean of anti-S IgG antibodies was 357 (95% CI, 96 to 1324) for participants receiving prednisone versus 2190 (CI, 1598 to 3002) for those not receiving it. Anti-S IgG antibody titers were also lower in those receiving B-cell depletion therapy (BCDT) (n = 10). Measures of immunogenicity differed numerically between those who were and those who were not receiving antimetabolites (n = 48), tumor necrosis factor inhibitors (n = 39), and Janus kinase inhibitors (n = 11); however, 95% CIs were wide and overlapped. Neutralization titers seemed generally consistent with anti-S IgG results. Results were not adjusted for differences in baseline clinical factors, including other immunosuppressant therapies.
Limitations: Small sample that lacked demographic diversity, and residual confounding.
Conclusion: Compared with nonusers, patients with CID treated with glucocorticoids and BCDT seem to have lower SARS-CoV-2 vaccine-induced antibody responses. These preliminary findings require confirmation in a larger study.
Primary funding source: The Leona M. and Harry B. Helmsley Charitable Trust, Marcus Program in Precision Medicine Innovation, National Center for Advancing Translational Sciences, and National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Conflict of interest statement
Figures





Update of
-
Glucocorticoids and B Cell Depleting Agents Substantially Impair Immunogenicity of mRNA Vaccines to SARS-CoV-2.medRxiv [Preprint]. 2021 Apr 9:2021.04.05.21254656. doi: 10.1101/2021.04.05.21254656. medRxiv. 2021. Update in: Ann Intern Med. 2021 Nov;174(11):1572-1585. doi: 10.7326/M21-1757. PMID: 33851176 Free PMC article. Updated. Preprint.
References
-
- Johns Hopkins Coronavirus Resource Center. Global Map. Accessed at https://coronavirus.jhu.edu on 18 June 2021.
-
- Voysey M , Clemens SAC , Madhi SA , et al; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99-111. [PMID: ] doi: 10.1016/S0140-6736(20)32661-1 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
