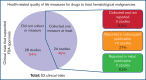
Assessment and reporting of quality-of-life measures in pivotal clinical trials of hematological malignancies
- PMID: 34461636
- PMCID: PMC8759126
- DOI: 10.1182/bloodadvances.2021004190
Assessment and reporting of quality-of-life measures in pivotal clinical trials of hematological malignancies
Figures
References
-
- US Department of Health and Human Services FDA Center for Drug Evaluation and Research, US Department of Health and Human Services FDA Center for Biologics Evaluation and Research, US Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual Life Outcomes. 2006;4(1):79. - PMC - PubMed
-
- US Food and Drug Administration. FDA Announces First of Its Kind Pilot Program to Communicate Patient Reported Outcomes from Cancer Clinical Trials. https://www.fda.gov/news-events/press-announcements/fda-announces-first-.... Accessed 2 February 2021.
-
- Esser P, Kuba K, Mehnert A, et al. Quality of life in survivors of hematological malignancies stratified by cancer type, time since diagnosis and stem cell transplantation. Eur J Haematol. 2018;101(3):340-348. - PubMed