Predisposition to Proinsulin Misfolding as a Genetic Risk to Diet-Induced Diabetes
- PMID: 34462258
- PMCID: PMC8564407
- DOI: 10.2337/db21-0422
Predisposition to Proinsulin Misfolding as a Genetic Risk to Diet-Induced Diabetes
Erratum in
-
Erratum. Predisposition to Proinsulin Misfolding as a Genetic Risk to Diet-Induced Diabetes. Diabetes 2021;70:2580-2594.Diabetes. 2022 Apr 1;71(4):870. doi: 10.2337/db22-er04a. Diabetes. 2022. PMID: 34994789 Free PMC article. No abstract available.
Abstract
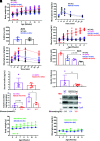
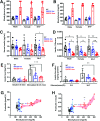
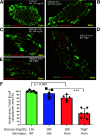
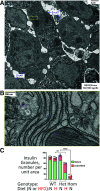
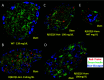
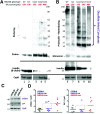
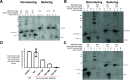
Throughout evolution, proinsulin has exhibited significant sequence variation in both C-peptide and insulin moieties. As the proinsulin coding sequence evolves, the gene product continues to be under selection pressure both for ultimate insulin bioactivity and for the ability of proinsulin to be folded for export through the secretory pathway of pancreatic β-cells. The substitution proinsulin-R(B22)E is known to yield a bioactive insulin, although R(B22)Q has been reported as a mutation that falls within the spectrum of mutant INS-gene-induced diabetes of youth. Here, we have studied mice expressing heterozygous (or homozygous) proinsulin-R(B22)E knocked into the Ins2 locus. Neither females nor males bearing the heterozygous mutation developed diabetes at any age examined, but subtle evidence of increased proinsulin misfolding in the endoplasmic reticulum is demonstrable in isolated islets from the heterozygotes. Moreover, males have indications of glucose intolerance, and within a few weeks of exposure to a high-fat diet, they developed frank diabetes. Diabetes was more severe in homozygotes, and the development of disease paralleled a progressive heterogeneity of β-cells with increasing fractions of proinsulin-rich/insulin-poor cells as well as glucagon-positive cells. Evidently, subthreshold predisposition to proinsulin misfolding can go undetected but provides genetic susceptibility to diet-induced β-cell failure.
© 2021 by the American Diabetes Association.
Figures

References
-
- Arneth B. Insulin gene mutations and posttranslational and translocation defects: associations with diabetes. Endocrine 2020;70:488–497 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

