Effects of Spironolactone and Chlorthalidone on Cardiovascular Structure and Function in Chronic Kidney Disease: A Randomized, Open-Label Trial
- PMID: 34462286
- PMCID: PMC8499017
- DOI: 10.2215/CJN.01930221
Effects of Spironolactone and Chlorthalidone on Cardiovascular Structure and Function in Chronic Kidney Disease: A Randomized, Open-Label Trial
Abstract
Background and objectives: In a randomized double-blind, placebo-controlled trial, treatment with spironolactone in early-stage CKD reduced left ventricular mass and arterial stiffness compared with placebo. It is not known if these effects were due to BP reduction or specific vascular and myocardial effects of spironolactone.
Design, setting, participants, & measurements: A prospective, randomized, open-label, blinded end point study conducted in four UK centers (Birmingham, Cambridge, Edinburgh, and London) comparing spironolactone 25 mg to chlorthalidone 25 mg once daily for 40 weeks in 154 participants with nondiabetic stage 2 and 3 CKD (eGFR 30-89 ml/min per 1.73 m2). The primary end point was change in left ventricular mass on cardiac magnetic resonance imaging. Participants were on treatment with an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker and had controlled BP (target ≤130/80 mm Hg).
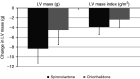
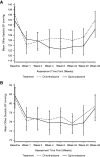
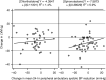
Results: There was no significant difference in left ventricular mass regression; at week 40, the adjusted mean difference for spironolactone compared with chlorthalidone was -3.8 g (95% confidence interval, -8.1 to 0.5 g, P=0.08). Office and 24-hour ambulatory BPs fell in response to both drugs with no significant differences between treatment. Pulse wave velocity was not significantly different between groups; at week 40, the adjusted mean difference for spironolactone compared with chlorthalidone was 0.04 m/s (-0.4 m/s, 0.5 m/s, P=0.90). Hyperkalemia (defined ≥5.4 mEq/L) occurred more frequently with spironolactone (12 versus two participants, adjusted relative risk was 5.5, 95% confidence interval, 1.4 to 22.1, P=0.02), but there were no patients with severe hyperkalemia (defined ≥6.5 mEq/L). A decline in eGFR >30% occurred in eight participants treated with chlorthalidone compared with two participants with spironolactone (adjusted relative risk was 0.2, 95% confidence interval, 0.05 to 1.1, P=0.07).
Conclusions: Spironolactone was not superior to chlorthalidone in reducing left ventricular mass, BP, or arterial stiffness in nondiabetic CKD.
Keywords: aldosterone; cardiovascular system; chlorthalidone; chronic kidney disease; randomized controlled trials; spironolactone.
Copyright © 2021 by the American Society of Nephrology.
Figures

References
-
- Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW; American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention : Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 42: 1050–1065, 2003 - PubMed
-
- Thomas B, Matsushita K, Abate KH, Al-Aly Z, Ärnlöv J, Asayama K, Atkins R, Badawi A, Ballew SH, Banerjee A, Barregård L, Barrett-Connor E, Basu S, Bello AK, Bensenor I, Bergstrom J, Bikbov B, Blosser C, Brenner H, Carrero JJ, Chadban S, Cirillo M, Cortinovis M, Courville K, Dandona L, Dandona R, Estep K, Fernandes J, Fischer F, Fox C, Gansevoort RT, Gona PN, Gutierrez OM, Hamidi S, Hanson SW, Himmelfarb J, Jassal SK, Jee SH, Jha V, Jimenez-Corona A, Jonas JB, Kengne AP, Khader Y, Khang YH, Kim YJ, Klein B, Klein R, Kokubo Y, Kolte D, Lee K, Levey AS, Li Y, Lotufo P, El Razek HMA, Mendoza W, Metoki H, Mok Y, Muraki I, Muntner PM, Noda H, Ohkubo T, Ortiz A, Perico N, Polkinghorne K, Al-Radaddi R, Remuzzi G, Roth G, Rothenbacher D, Satoh M, Saum KU, Sawhney M, Schöttker B, Shankar A, Shlipak M, Silva DAS, Toyoshima H, Ukwaja K, Umesawa M, Vollset SE, Warnock DG, Werdecker A, Yamagishi K, Yano Y, Yonemoto N, Zaki MES, Naghavi M, Forouzanfar MH, Murray CJL, Coresh J, Vos T; Global Burden of Disease 2013 GFR Collaborators; CKD Prognosis Consortium; Global Burden of Disease Genitourinary Expert Group : Global cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol 28: 2167–2179, 2017 - PMC - PubMed
-
- Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, Matsushita K, Wen CP: Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 382: 339–352, 2013 - PubMed
-
- Edwards NC, Moody WE, Chue CD, Ferro CJ, Townend JN, Steeds RP: Defining the natural history of uremic cardiomyopathy in chronic kidney disease: The role of cardiovascular magnetic resonance. JACC Cardiovasc Imaging 7: 703–714, 2014 - PubMed
-
- Bomback AS, Klemmer PJ: The incidence and implications of aldosterone breakthrough. Nat Clin Pract Nephrol 3: 486–492, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

