SNX27-FERM-SNX1 complex structure rationalizes divergent trafficking pathways by SNX17 and SNX27
- PMID: 34462354
- PMCID: PMC8433557
- DOI: 10.1073/pnas.2105510118
SNX27-FERM-SNX1 complex structure rationalizes divergent trafficking pathways by SNX17 and SNX27
Abstract
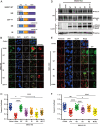
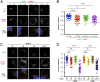
The molecular events that determine the recycling versus degradation fates of internalized membrane proteins remain poorly understood. Two of the three members of the SNX-FERM family, SNX17 and SNX31, utilize their FERM domain to mediate endocytic trafficking of cargo proteins harboring the NPxY/NxxY motif. In contrast, SNX27 does not recycle NPxY/NxxY-containing cargo but instead recycles cargo containing PDZ-binding motifs via its PDZ domain. The underlying mechanism governing this divergence in FERM domain binding is poorly understood. Here, we report that the FERM domain of SNX27 is functionally distinct from SNX17 and interacts with a novel DLF motif localized within the N terminus of SNX1/2 instead of the NPxY/NxxY motif in cargo proteins. The SNX27-FERM-SNX1 complex structure reveals that the DLF motif of SNX1 binds to a hydrophobic cave surrounded by positively charged residues on the surface of SNX27. The interaction between SNX27 and SNX1/2 is critical for efficient SNX27 recruitment to endosomes and endocytic recycling of multiple cargoes. Finally, we show that the interaction between SNX27 and SNX1/2 is critical for brain development in zebrafish. Altogether, our study solves a long-standing puzzle in the field and suggests that SNX27 and SNX17 mediate endocytic recycling through fundamentally distinct mechanisms.
Keywords: SNX27; endosomal sorting; endosome; membrane trafficking; sorting nexin.
Conflict of interest statement
The authors declare no competing interest.
Figures





References
-
- Cullen P. J., Steinberg F., To degrade or not to degrade: Mechanisms and significance of endocytic recycling. Nat. Rev. Mol. Cell Biol. 19, 679–696 (2018). - PubMed
-
- Small S. A., Petsko G. A., Retromer in Alzheimer disease, Parkinson disease and other neurological disorders. Nat. Rev. Neurosci. 16, 126–132 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

