Neighboring Pd single atoms surpass isolated single atoms for selective hydrodehalogenation catalysis
- PMID: 34462434
- PMCID: PMC8405729
- DOI: 10.1038/s41467-021-25526-2
Neighboring Pd single atoms surpass isolated single atoms for selective hydrodehalogenation catalysis
Abstract
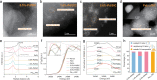
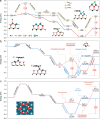
Single atom catalysts have been found to exhibit superior selectivity over nanoparticulate catalysts for catalytic reactions such as hydrogenation due to their single-site nature. However, improved selectively is often accompanied by loss of activity and slow kinetics. Here we demonstrate that neighboring Pd single atom catalysts retain the high selectivity merit of sparsely isolated single atom catalysts, while the cooperative interactions between neighboring atoms greatly enhance the activity for hydrogenation of carbon-halogen bonds. Experimental results and computational calculations suggest that neighboring Pd atoms work in synergy to lower the energy of key meta-stable reactions steps, i.e., initial water desorption and final hydrogenated product desorption. The placement of neighboring Pd atoms also contribute to nearly exclusive hydrogenation of carbon-chlorine bond without altering any other bonds in organohalogens. The promising hydrogenation performance achieved by neighboring single atoms sheds light on a new approach for manipulating the activity and selectivity of single atom catalysts that are increasingly studied in multiple applications.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Wang A, Li J, Zhang T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018;2:65–81. doi: 10.1038/s41570-018-0010-1. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

