Microbial production of megadalton titin yields fibers with advantageous mechanical properties
- PMID: 34462443
- PMCID: PMC8405620
- DOI: 10.1038/s41467-021-25360-6
Microbial production of megadalton titin yields fibers with advantageous mechanical properties
Abstract
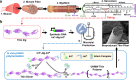
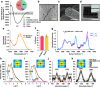
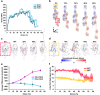
Manmade high-performance polymers are typically non-biodegradable and derived from petroleum feedstock through energy intensive processes involving toxic solvents and byproducts. While engineered microbes have been used for renewable production of many small molecules, direct microbial synthesis of high-performance polymeric materials remains a major challenge. Here we engineer microbial production of megadalton muscle titin polymers yielding high-performance fibers that not only recapture highly desirable properties of natural titin (i.e., high damping capacity and mechanical recovery) but also exhibit high strength, toughness, and damping energy - outperforming many synthetic and natural polymers. Structural analyses and molecular modeling suggest these properties derive from unique inter-chain crystallization of folded immunoglobulin-like domains that resists inter-chain slippage while permitting intra-chain unfolding. These fibers have potential applications in areas from biomedicine to textiles, and the developed approach, coupled with the structure-function insights, promises to accelerate further innovation in microbial production of high-performance materials.
© 2021. The Author(s).
Conflict of interest statement
C.H.B., C.J.S. and F.Z. have filed a provisional patent application (# 63/113267) based on this work. All other authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

