NTS Prlh overcomes orexigenic stimuli and ameliorates dietary and genetic forms of obesity
- PMID: 34462445
- PMCID: PMC8405610
- DOI: 10.1038/s41467-021-25525-3
NTS Prlh overcomes orexigenic stimuli and ameliorates dietary and genetic forms of obesity
Abstract
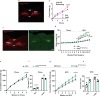
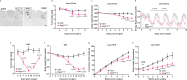
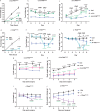
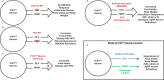
Calcitonin receptor (Calcr)-expressing neurons of the nucleus tractus solitarius (NTS; CalcrNTS cells) contribute to the long-term control of food intake and body weight. Here, we show that Prlh-expressing NTS (PrlhNTS) neurons represent a subset of CalcrNTS cells and that Prlh expression in these cells restrains body weight gain in the face of high fat diet challenge in mice. To understand the relationship of PrlhNTS cells to hypothalamic feeding circuits, we determined the ability of PrlhNTS-mediated signals to overcome enforced activation of AgRP neurons. We found that PrlhNTS neuron activation and Prlh overexpression in PrlhNTS cells abrogates AgRP neuron-driven hyperphagia and ameliorates the obesity of mice deficient in melanocortin signaling or leptin. Thus, enhancing Prlh-mediated neurotransmission from the NTS dampens hypothalamically-driven hyperphagia and obesity, demonstrating that NTS-mediated signals can override the effects of orexigenic hypothalamic signals on long-term energy balance.
© 2021. The Author(s).
Conflict of interest statement
CJR is an employee of and holds stock in AstraZeneca. DPO and MGM, Jr. receive research funding from AstraZeneca and Novo Nordisk. The other authors declare that they have no conflicts of interest relevant to this paper.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

