This is a preprint.
The B.1.427/1.429 (epsilon) SARS-CoV-2 variants are more virulent than ancestral B.1 (614G) in Syrian hamsters
- PMID: 34462750
- PMCID: PMC8404898
- DOI: 10.1101/2021.08.25.457626
The B.1.427/1.429 (epsilon) SARS-CoV-2 variants are more virulent than ancestral B.1 (614G) in Syrian hamsters
Update in
-
The B.1.427/1.429 (epsilon) SARS-CoV-2 variants are more virulent than ancestral B.1 (614G) in Syrian hamsters.PLoS Pathog. 2022 Feb 10;18(2):e1009914. doi: 10.1371/journal.ppat.1009914. eCollection 2022 Feb. PLoS Pathog. 2022. PMID: 35143587 Free PMC article.
Abstract
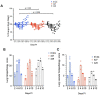
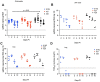
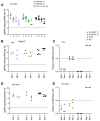
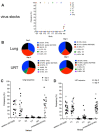
As novel SARS-CoV-2 variants continue to emerge, it is critical that their potential to cause severe disease and evade vaccine-induced immunity is rapidly assessed in humans and studied in animal models. In early January 2021, a novel variant of concern (VOC) designated B.1.429 comprising 2 lineages, B.1.427 and B.1.429, was originally detected in California (CA) and shown to enhance infectivity in vitro and decrease antibody neutralization by plasma from convalescent patients and vaccine recipients. Here we examine the virulence, transmissibility, and susceptibility to pre-existing immunity for B 1.427 and B 1.429 in the Syrian hamster model. We find that both strains exhibit enhanced virulence as measured by increased body weight loss compared to hamsters infected with ancestral B.1 (614G), with B.1.429 causing the most body weight loss among all 3 lineages. Faster dissemination from airways to parenchyma and more severe lung pathology at both early and late stages were also observed with B.1.429 infections relative to B.1. (614G) and B.1.427 infections. In addition, subgenomic viral RNA (sgRNA) levels were highest in oral swabs of hamsters infected with B.1.429, however sgRNA levels in lungs were similar in all three strains. This demonstrates that B.1.429 replicates to higher levels than ancestral B.1 (614G) or B.1.427 in the upper respiratory tract (URT) but not in the lungs. In multi-virus in-vivo competition experiments, we found that epsilon (B.1.427/B.1.429) and gamma (P.1) dramatically outcompete alpha (B.1.1.7), beta (B.1.351) and zeta (P.2) in the lungs. In the URT gamma, and epsilon dominate, but the highly infectious alpha variant also maintains a moderate size niche. We did not observe significant differences in airborne transmission efficiency among the B.1.427, B.1.429 and ancestral B.1 (614G) variants in hamsters. These results demonstrate enhanced virulence and high relative fitness of the epsilon (B.1.427/B.1.429) variant in Syrian hamsters compared to an ancestral B.1 (614G) strain.
Author summary: In the last 12 months new variants of SARS-CoV-2 have arisen in the UK, South Africa, Brazil, India, and California. New SARS-CoV-2 variants will continue to emerge for the foreseeable future in the human population and the potential for these new variants to produce severe disease and evade vaccines needs to be understood. In this study, we used the hamster model to determine the epsilon (B.1.427/429) SARS-CoV-2 strains that emerged in California in late 2020 cause more severe disease and infected hamsters have higher viral loads in the upper respiratory tract compared to the prior B.1 (614G) strain. These findings are consistent with human clinical data and help explain the emergence and rapid spread of this strain in early 2021.
Figures




Similar articles
-
The B.1.427/1.429 (epsilon) SARS-CoV-2 variants are more virulent than ancestral B.1 (614G) in Syrian hamsters.PLoS Pathog. 2022 Feb 10;18(2):e1009914. doi: 10.1371/journal.ppat.1009914. eCollection 2022 Feb. PLoS Pathog. 2022. PMID: 35143587 Free PMC article.
-
Emerging Variants of SARS-CoV-2 and Novel Therapeutics Against Coronavirus (COVID-19).2023 May 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2023 May 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 34033342 Free Books & Documents.
-
Characterization of a new SARS-CoV-2 variant that emerged in Brazil.Proc Natl Acad Sci U S A. 2021 Jul 6;118(27):e2106535118. doi: 10.1073/pnas.2106535118. Proc Natl Acad Sci U S A. 2021. PMID: 34140350 Free PMC article.
-
The Biological Functions and Clinical Significance of SARS-CoV-2 Variants of Corcern.Front Med (Lausanne). 2022 May 20;9:849217. doi: 10.3389/fmed.2022.849217. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35669924 Free PMC article. Review.
-
Variants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Vaccine Effectiveness.Vaccines (Basel). 2022 Oct 19;10(10):1751. doi: 10.3390/vaccines10101751. Vaccines (Basel). 2022. PMID: 36298616 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
