Clinical outcomes of NSCLC patients experiencing early immune-related adverse events to PD-1/PD-L1 checkpoint inhibitors leading to treatment discontinuation
- PMID: 34462870
- PMCID: PMC10991137
- DOI: 10.1007/s00262-021-03045-9
Clinical outcomes of NSCLC patients experiencing early immune-related adverse events to PD-1/PD-L1 checkpoint inhibitors leading to treatment discontinuation
Abstract
Background: The prognostic relevance of early immune-related adverse events (irAEs) in patients affected by non-small cell lung cancer (NSCLC) upon immunotherapy is not fully understood.
Methods: The Leading to Treatment Discontinuation cohort included 24 patients experiencing severe irAEs after one of two administrations of single anti-PD-1/PD-L1 in any line setting for metastatic NSCLC between November 2015 and June 2019. The control cohort was composed of 526 patients treated with single anti-PD-1/PD-L1 in any line setting with no severe irAE reported. The primary end points were median progression-free survival, overall survival, objective response rate, risk of progression of disease and risk of death. The correlation of clinic pathological features with early severe irAEs represented the secondary end point.
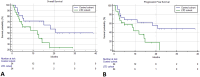
Results: Median PFS was 9.3 and 8.4 months, median OS was 12.0 months and 14.2 months at a median follow-up of 18.1 and 22.6 months in the LTD cohort and in the control cohort, respectively. The ORR was 40% (95% CI 17.2-78.8) in the LTD cohort and 32.7% (95% CI 27.8-38.2) in the control cohort. The risk of disease progression was higher in the LTD cohort (HR 2.52 [95% 1.10-5.78], P = .0288).
Conclusions: We found no survival benefit in LTD cohort compared to the control cohort. However, early and severe irAEs might underly an immune anti-tumor activation. We identified a significant association with first-line immune checkpoints inhibitors treatment and good PS. Further studies on risk prediction and management of serious and early irAEs in NSCLC patients are needed.
Keywords: Early immune-related adverse events; Immune checkpoints inhibitors; Non-small cell lung cancer; Prediction.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Alessio Cortellini received speaker fees and grant consultancies from Roche, MSD, BMS, AstraZeneca, Novartis, Astellas. Marco Russano received honoraria for consultancy and scientific talks from Roche, BMS, MSD, Boehringer Ingelheim, AstraZeneca. Daniele Santini received speaker fees and grant consultancies from Janssen, Astellas, BMS, MSD, Pfizer, Boeringher Ingelheim, Roche, Eisai, Incyte, Novartis. Marcello Tiseo received speakers’ and consultants’ fee from AstraZeneca, Pfizer, Eli-Lilly, BMS, Novartis, Roche, MSD, Boehringer Ingelheim, Otsuka, Takeda, Pierre Fabre. Marcello Tiseo received institutional research grants from AstraZeneca, Boehringer Ingelheim. Sebastiano Buti received honoraria as a speaker at scientific events and advisory role by Bristol-Myers Squibb (BMS), Pfizer; MSD, Ipsen, Roche, Eli-Lilly, AstraZeneca, Pierre Fabre and Novartis.
Figures
References
-
- Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB (2017) Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol 8:49. 10.3389/fphar.2017.00049 [published correction appears in Front Pharmacol 2017 8:311. 10.3389/fphar.2017.00311]
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials