Take a "Snapshot" of New Syntheses, Reactions, and Characterizations from Unusual Unsaturated Ring Strained Group 4 Metallacycles
- PMID: 34463390
- PMCID: PMC9293241
- DOI: 10.1002/chem.202102855
Take a "Snapshot" of New Syntheses, Reactions, and Characterizations from Unusual Unsaturated Ring Strained Group 4 Metallacycles
Abstract
Recently published syntheses, reactions and characterizations of unusual unsaturated ring strained Group 4 metallocene metallacycles like metalla-cyclocumulenes, -cycloallenes and -cycloalkynes with different ring size are updated for the last three years. There exist for some of these metallacycles, depending on the ring size, 7-, 5- and 4-membered compounds. The new results for these metallacycles are summarized here and considered in addition to the former published results. Additionally, several compounds of this type were now characterized by new reactions. For a better understanding of these compounds, some spectroscopical methods as well as theoretical calculations were published. Despite of these all-C-metallacycles, only in some cases the syntheses and reactions for the corresponding hetero-metallacycles were published too. Examples for these metallaheterocyclic compounds will not be considered in this article. All these unusual ring strained compounds have a great potential for a lot of interesting synthetic applications in the future. Additionally, they are very interesting from the theoretical point of view.
Keywords: group 4 metals; organic syntheses; organometallic compounds; theoretical investigations; unusual metallacycles.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
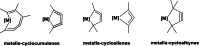






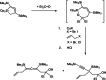



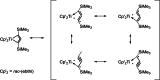
References
-
- Selected Reviews:
-
- Ohff A., Pulst S., Lefeber C., Peulecke N., Arndt P., Burlakov V. V., Rosenthal U., Synlett 1996, 111–118;
-
- Rosenthal U., Burlakov V. V., Arndt P., Baumann W., Spannenberg A., Organometallics 2005, 24, 456–471;
-
- Rosenthal U., Burlakov V. V., Bach M. A., Beweries T., Chem. Soc. Rev. 2007, 36, 689–820; - PubMed
-
- Rosenthal U., Angew. Chem. 2008, 120, 5196–5199;
- Angew. Chem. Int. Ed. 2008, 47, 5118–5121; - PubMed
Publication types
LinkOut - more resources
Full Text Sources

